IJCRR - 7(2), January, 2015
Pages: 61-65
Print Article
Download XML Download PDF
CORRELATION BETWEEN BIOFILM FORMATION AND HIGHLY DRUG RESISTANT UROPATHOGENS (HDRU)
Author: V. S. Deotale, Ruchita Attal, S. Joshi, N. Bankar
Category: Healthcare
Abstract:Background: The biofilms have a major medical significance as they decrease the susceptibility to the antimicrobial agents. Furthermore, the proximity of cells within a biofilm can facilitate a plasmid exchange and hence enhance the spread of antimicrobial
resistance.
Objectives: The present study intends to detect biofilm formation and High Drug Resistance amongst the uropathogens and to correlate between biofilm formation and HDRU.
Methods: This cross-sectional study was carried out over a period of two months, including 37 catheterized urinary isolate with symptoms of UTI. Following their identification, these isolates were checked for biofilm formation by three different phenotypic
methods which includes tube adherence, Congo red agar method & tissue culture plate method. Antibiotic Susceptibility Test
was done by Kirby \? Bauer Disk Diffusion method as per CLSI guidelines.
Results: Out of total 37 uropathogens isolated from catheterized urine samples, 30 (81.1%) were positive in vitro for biofilm
production & 22 (59.5%) isolates were HDRU. Maximum biofilm production was shown by E.coli (50%) , followed by Klebsiella
pneumoniae (33.3%).
Keywords: Tissue Culture Plate Method (TCP), Catheter Associated Urinary Tract Infections (CAUTI), Congo red agar method
Full Text:
INTRODUCTION
Biofilms are a population of multilayered cells growing on a surface . These cells have a layer of adhesins in their cell walls that allow them to colonize many types of substrate, and, on contact with a surface, the cells secrete exopolysaccharides that secure their attachment. The bacteria multiply to form microcolonies of cells that subsequently spread over the surface, forming populations embedded in a gel-like polysaccharide matrix. It was found that the major pathogenic factor is the ability to form biofilm on polymeric surfaces to which it adheres and colonizes artificial materials 1 . The biofilms play major role in decreasing the susceptibility to the antimicrobial agents; as the proximity of cells within a biofilm can facilitate a plasmid exchange and hence enhance the spread of antimicrobial resistance.2 Microorganisms that are apparently fully sensitive to antibiotics and antiseptics in conventional laboratory testing methods become fully resistant in the biofilm mode in vivo3 . Microbial biofilms are considered as the major problem in catheterized patients because they cause chronic infections which are difficult to treat, lead to longer hospitalization time, and can result in much higher treatment costs 4 . Urinary tract infections in catheterized patients can occur in several ways. Organisms that colonize the periurethral skin can migrate into the bladder through the mucoid film that forms between the epithelial surface of the urethra and the catheter. In addition, contamination of the urine in the drainage bag can allow organisms to access the bladder through the drainage tube and the catheter lumen.5,6 Importance of correlation between biofilm production and Highly Drug Resistant Uropathogens ( HDRU) makes the therapeutic options very limited, have large impact on the empirical therapy to newer and more potent antimicrobials. Urinary Gram Negative Rods (GNRs) that are resistant to third generation Cephalosporins , Ciprofloxacin and Gentamicin/ Amikacin are defined as highly drug resistant uropathogens (HDRU) 7 . Keeping this in view the present study was conducted with following objectives;
OBJECTIVES
1. To detect biofilm formation by the tube adherence method(TA)8,9, Congo Red agar method (CRA)10,11 and Tissue culture plate method( TCP)12.
2. To correlate biofilm formation with development of high drug resistance amongst the uropathogens (HDRU).
MATERIAL & METHODS
The study was conducted in a period of 2 months duration in the department of Microbiology, a tertiary care rural hospital in Central India. A total of 37 bacterial isolates obtained from catheterized urine samples of catheter associated urinary tract infection (CAUTI) were included in the study. Urine samples were collected from indoor patients with a urinary catheter for at least 2 days suffering from symptoms of UTI like fever> 380 C, urgency, frequency, dysuria or suprapubic tenderness. Samples were collected under all aseptic precaution with sterile syringe from the distal end of urinary catheter into a sterile container and transported immediately to the laboratory. The urine samples were inoculated with the help of sterile inoculating calibrated standard loop of 4mm inside diameter onto Blood Agar, MacConkey’s agar and the Cystine Lactose Electrolyte Deficient (CLED) medium to determine the Colony Forming Units (CFU).. All Isolates were identified by standard microbiological procedures13. Reference strain of positive biofilm producer Staphylococcus epidermidis ATCC 35984, Staphylococcus aureus ATCC 25923 (non-slime producer) were used as a control.
Biofilm detection:
The detection of the biofilms was done by the tube adherence method(TA)8,9,Congo Red agar method (CRA)10,11 and Tissue culture plate method( TCP)12. Urine samples were collected from indoor patients with urinary catheters since 48 hours and symptoms of UTI.
TA8,9:
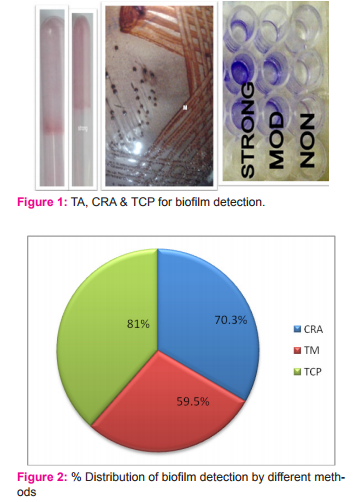
The investigation of the biofilm production was done on the basis of the adherence of the biofilms to borosilicate test tubes, as was done by Christensen et al. (1982)8 Suspensions of the tested strains were incubated in glass tubes which contained Brain Heart Infusion Broth (broth) aerobically at a temperature of 35°C for a period of 2 days. Then, the supernatants were discarded and the glass tubes were stained with a 0.1% Safranin solution, washed with distilled water 3 times and dried. A positive result was interpreted as the presence of a layer of a stained material which adhered to the inner wall of the tubes. The exclusive observation of a stained ring at the liquid-air interface was considered as negative9 [Fig-1].
CRA10,11:
The suspensions of the tested strains were inoculated into tubes which contained a specially prepared solid medium- Brain Heart Infusion broth (BHI) which was supplemented with 5% sucrose and Congo Red. The medium was composed of BHI (37 gms/L), sucrose (50 gms/L), agar no.1 (10 gms/L) and the Congo Red stain (0.8 gms/L). The plates were inoculated and incubated aerobically for 24-48 hours at 37°C. A positive result was indicated by black colonies with a dry crystalline consistency [Fig-2]. A darkening of the colonies, with the absence of a dry crystalline colonial morphology, indicated an indeterminate result11. The experiment was performed in triplicate and it was repeated 3 times.
TCP 12,14:
All isolates were screened for their ability to form biofilm by the TCP method as described by Christensen et al. 12 with a modification in duration of incubation which was extended to 24 hours, according to O’Toole and Kolter 14. Isolates from fresh agar plates were inoculated in trypticase soy broth with 1% glucose and incubated for 24 hours at 37o C in stationary condition and diluted (1 in 100) with fresh medium. Individual wells of sterile, polystyrene, flat-bottom tissue culture plates were filled with 0.2 ml aliquots of the diluted cultures, and only broth served as control to check for the sterility and non-specific binding of media. The tissue culture plates were incubated for 24 hours at 37°C. After incubation, the content of each well was gently removed by tapping the plates. The wells were washed four times with 0.2 ml of phosphate buffer saline (PBS pH 7.2) to remove freefloating planktonic bacteria; then 25 µl of 1% solution of crystal violet was added to each well (this dye stains the cells but not the polystyrene) plates. The plates were incubated at room temperature for 15 minutes, rinsed thoroughly and repeatedly with water. Adherent cells were uniformly stained with crystal violet which was solubilized in 200 µl of 95 % ethanol ; of which 125 µl were transferred to a new polystyrene microtiter dish, which was then read15. Optical densities (OD) of stained adherent bacteria were determined with a micro ELISA auto reader (model 680, Bio rad), and the wavelength of values was considered as an index of bacteria adhering to surface and forming biofilms. Experi-ments for each strain were performed in triplicate and repeated three times. The OD of each well was measured at 578 nm using ELISA reader. Biofilm production is considered high, moderate, or weak (OD570 nm)15.
RESULTS
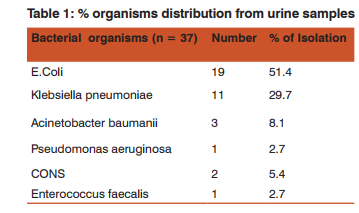
Amongst the 37 bacterial isolates from the CAUTI, E.coli were found to be 51.4% followed by Klebsiella pneumoniae 29.7% , A. baumanii 8.1% ,CONS 5.4% , Pseudomonas aeruginosa and E. faecalis 2.7% each (Table 1). In the present study, 30 (81%) isolates were in vitro positive for the biofilm production and 7 (19%) were negative for biofilm production. Maximum biofilm production was shown by E.coli (50%) , followed by Klebsiella pneumoniae (33.3%) (Table 2). The results for biofilm production with CRA was 70.3% followed by TA 59.5% and by TCP 81%. There was complete agreement for biofilm production in 54% isolates by all the three methods. In this study, out of 37 isolates, 26 (70.3%) were slime producers developing almost black or very black colonies on CRA plate as presented in Figure 1 and the remaining 11 were non-producers developing red colonies. 22 strains (59.5%) revealed in vitro biofilm formation by TA method. (Table3) In our study, biofilm formation and HDRU were mostly isolated from medicine Intensive Care Unit & Paediatrics Intensive care unit (MICU and PICU). (Table 4). We have observed higher drug resistance uropathogens in highest number (32%) in patients who were catheterized for acute retention of urine associated with Benign Prostatic hypertrophy(BPH) followed by post-operative catheterization(28%). Most of the biofilm formation was observed in those who were catheterized post-operatively (40%) in cases of DUB, Ca Cervix, Prolapse uterus, fibroid uterus, post-partam eclampsia.
Statistical Analysis of TCP, TM and CRA methods:
Considering TCP method as a gold standard for this study sensitivity data was compared with the data from tube method and CRA method. Parameters like sensitivity, specificity, negative predictive value and positive predictive value were calculated. Sensitivity & specificity of TM was found to be 70% and 85.7% respectively while that of CRA was 80% and 71.4%.
DISCUSSION
Bacteria have a basic survival strategy to colonize surface and grows as biofilm communities embedded in gel like polysaccharide matrix. The catheterized urinary tract provides ideal conditions for the development of enormous biofilm population and induces problems in patient’s management. Clinical prevention strategies are needed, as bacteria growing in the biofilm mode are resistant to antibiotics.CAUTI is the most common nosocomial infection in hospitals and comprising >40% of all institutionally acquired infections9 . The correlation between biofilm formation & CAUTI is that a foreign body, such as an indwelling urethral catheter, connects a normally sterile hydrated body site to the outside world which inevitably become colonized with microorganisms. In our study, out of 37 isolates, E.coli was found to be the most frequently isolated uropathogen 51.4% , followed by Klebsiella pneumoniae 29.7%, Acinetobacter baumanii 8.1%, Coagulase Negative Staphylococcus 5.4% and Enterococcus fecalis and Pseudomonas aeruginosa 2.7% each. Pramodini et al9 in her study found E.coli (70%) remains the predominant uropathogen isolated from CAUTI. Hassin et al16 also reported predominant uropathogen from catheterized urine as E.coli (74%) followed by Klebsiella spp. (17.7%) and Pseudomonas spp. (2.5%). The current study reveals 81.1% of strains were in vitro positive for biofilm production. Reid et al17 reported 73% biofilm production by uropathogens from UTI. Significant production of biofilm was seen in E.coli (50%) followed by, Klebsiella pneumoniae (33.3%) which is similar to Pramodini et al9 (63%) and Sharma et al18 (70.3%). Amongst 30 biofilm producing isolates, 20 (66.7%) isolates were biofilm producer by all the three methods and 21 (70%) isolates were biofilm producers by TA, 24(80%) by CRA and 30(100%) by TCP. Antimicrobial resistance is an innate feature of bacterial biofilms that may further complicate patient treatment. We also studied the antibiotic susceptibility pattern of all uropathogens and correlated that with biofilm production. In this study, 22 (59.5%) isolates were HDRU while 15(40.5%) were non-HDRU. Similar to biofilm production, maximum HDRU were E.coli 11(50%) followed by Klebsiella pneumonia 8( 36.7%). Out of 30 biofilm producing isolates 20(66.6%) were Highly Drug Resistance Uropathogens. Amongst 22 HDRU, 20 (90%) were biofilm producers. Sanchez et al19 also observed that strains capable of forming biofilms were more frequently observed to be an MDR phenotype. Significant bacteriuria was present in all symptomatic catheterized patients and E.coli was the most common uropathogen.
CONCLUSION
It has been shown that there is significant association between biofilm production and Highly Drug Resistant Uropathogens. Tissue culture plate method is the gold standard for detection of biofilm formation. Proper management of patients and implementation of standard guidelines for care of catheters to prevent the device associated nosocomial infections. The future goal is to identify molecular targets of biofilm bacteria as well as the urinary components that are involved in biofilm formation. An ideal surface device to resist protein has to be developed. Bacterial adhesion and the interaction between the biomaterial surface and urine also need to be defined.
ACKNOWLEDGEMENT
We kindly acknowledge the immense help received from scholars whose articles are cited and included in reference of this manuscript. We are also grateful to Authors/ Editors/ Publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Kloos WE, and Bannerman TL (1994) Update on clinical significance of coagulase–negative Staphylococci. Clin Microbiol Rev 7: 117–40.
2. Watnick P, Kotler R. Biofilm,city of microbes. J.Bacteriol 2000; 182: 2675–2679.
3. David J Stickler. Bacterial biofilms in patients with indwelling urinary catheters. Nature clinical practice urology November 2008 vol 5 no 11.
4. Desgrandchamps F, Moulinier F, Doudon M, Teillac P, Le Duc A. (1997) An in-vitro comparison of urease induced encrustation of JJ stents in human urine. Br J Urol 79: 24
5. Stamm WE (1991) Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med 91 (Suppl 3B): 65S–71S
6. Tambyah PA et al. (1999) A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc 74:131–136
7. Basak, S Bose, S Mallick, R Attal Highly Drug Resistant Uropathogens (HDRU) and their Antibiotic Susceptibility Profile – A case study. Ind Med Gaze, 2009 May;. (5):182- 5.
8. Christensen GD, Simpson WA, Bismo AL, Beachery EH. The adherence of the slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect immune 1982; 37: 318-26.
9. Pramodini S et al. Antiobiotic resistance pattern of biofilmforming uropathogens isolated from catheterized patients in Pondicherry, India. AMJ 2012, 5, 7, 344–348. http// dx.doi.org/10.4066/AMJ.2012.1193
10. Freeman DJ, Falkiner FR, Keane CT. A new method for the detection of the slime production by the coagulase negative Staphylococci. J Clin Pathol 1989; 42: 872-74
11. Niveditha S et al. The isolation and Biofilm Formation of Uropathogens in the patients with Catheter Associated Urinary Tract Infections (UTIs). Journal of Clinical and Diagnostic Research. 2012 November, Vol-6(9): 1478-1482
12. Christensen GD, Simpson WA, Younger JA, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985) Adherence of coagulase negative Staphylococci to plastic tissue cultures: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22: 996-1006.
13. Collee J G., Miles RS, Watt B. Test for identification of bacteria, in Chapter: 7 Mackie & McCartney’s Practical Medical Microbiology14th ed. In: JG Collee, AG Fraser, BP Marmion, A Simmons, Editors. Churchill Livingstone: Indian Reprint; 2008. p. 131-49.
14. O’Toole AG and Kolter R (1998) Initiation of biofilm formation in Pseudomonas fluorescence WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Molecular microbiology 28: 449.
15. Gad et al. Detection of icaA, icaD genes and biofilm production by Staphylococcus aureus and Staphylococcus epidermidis isolated from urinary tract catheterized patients. J Infect Dev Ctries 2009; 3(5):342-351.
16. Hassin SKR. Studies on Urinary Tract Infections. Bangladesh Medical Journal 1991; 20: 29–32.
17. Reid G, Charbonneau–Smith R, Lam D, Kang YS, Lacerte M, Hayes KC. Bacterial biofilm formation in the urinary bladder of spinalcord injured patients. Paraplegia. 1992; 30:711–717.
18. Sharma M, Aparna, Yadav S, Chaudhary U. Biofilm production in uropathogenic Escherichia coli. Indian J Pathol Microbiol 2009; 52: 294.
19. Sanchez et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infectious Diseases 2013, 13:47.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License