IJCRR - 7(2), January, 2015
Pages: 06-16
Print Article
Download XML Download PDF
METAL COMPLEXES OF SCHIFF BASE DERIVED FROM SALICYLALDEHYDE - A REVIEW
Author: K. A. Maher, S. R. Mohammed
Category: General Sciences
Abstract:Salicyaldehyde and their derivatives can be condensed with amines in 1:1 and 2:1 ratio to form bi, tri and tetra dentate NO and N2O2 Schiff base Ligands. They can contain additional donor atoms like oxygen, sulphur, nitrogen etc. which makes them Suitable chelating ligands to coordinate with Metal ions to form Schiff base complexes. Thus provides the possibility synthesis of large number of Schiff base complexes with divers' structural feature. The model system including those with bidentate, tridentate and tetra dentate Schiff base derived from Salicyaldehyde and their coordination chemistry are summarized in this review.
Keywords: Metal complexes, salicylaldehyde, Schiff Bases, Bidentate, Tridentate, Tetra dentate
Full Text:
INTRODUCTION
Salicylaldehyde
Salicylaldehyde is a key precursor to a variety chelating agent, some of which are commercially important, salicylaldehyde is a common highly functionalized arene that has often been exploited as a precursor to still other chemical. Salicylaldehyde is converted to chelating ligands by condensation with amines. With ethylenediamine, it condenses to give the ligand salen. Hydroxylamine gives salicylaldoxime. Oxidation with hydrogen peroxide gives catechol (1,2-dihydroxybenzene) (Dakin reaction)1 . {Dakin, 1923 #47}Condensation with diethyl malonate gives a derivative of the heterocycle coumarin 2 via an aldol condensation.
Schiff bases
Schiff bases are aldehyde- or ketone-like compounds in which the carbonyl group is replaced by an imine or azomethine group3 .Schiff bases are versatile ligands synthesized from the condensation of an amino compound with carbonyl compounds4,5,6 and were first reported by Hugo Schiff in 1864. Formation of Schiff base generally takes place under acid or base catalysis or with heat. The common Schiff bases are crystalline solids, which are feebly basic but at least some form insoluble salts with strong acids5 . Today, Schiff bases are used as intermediates for the synthesis of amino acids or as ligands for preparation of metal complexes having a series of different structures5.
Schiff base metal complexes
Schiff bases are the most widely used organic compounds7 for industrial purposes and also exhibit a broad range of biological activities.3 Schiff base compounds and their metal complexes are very important as catalysts in various biological systems, polymers, dyes and medicinal and pharmaceutical fields4,8 they comprise miscellaneous therapeutically potent applications in the field of medicinal chemistry.9 Their use in birth control, food packages and as an O2 detector is also outlined8 Schiff’s bases chelates also used in quantitative analysis as an analytical chemical reagents and/or separation reagents have been also listed and discussed6 and synthetic applications in the field of the organic and inorganic chemistry.9 They have been shown to exhibit a broad range of biological activities, including antifungal, antibacterial, antimalarial, antiproliferative, anti-inflammatory, antiviral, and antipyretic properties.3,7
Schiff’s base and their copper complexes possess remarkable properties as catalysts in various biological systems, polymers, dyes, antimicrobial activities, antifungal activities, antiviral activities insecticides, antitumor and cytotoxic activities, plant growth regulator, enzymatic activity and pharmaceutical fields.A variety of Schiff’ base and its complex shave been studied extensively.Several model systems, including those with bidentate, tridentate, tetradentate, multidentate Schiff base ligands, and their coordination chemistry of copper attracts much attention because of its biological relevance and its own interesting coordination chemistry such as geometry, flexible redox property, and oxidation state.4 Gou and coworker used Salicylaldehyde base-Schiff as novel, easily available colorimetric and fluorescent double-sensor. The sensor exhibits highly selective and sensitive recognition toward Cu+2 in aqueous solution via a naked eye color change from colorless to yellow and toward Al+3 via a significant fluorescent enhancement in ethanol over a wide range of tested metal ions. This represents the first reported Salicylaldehyde Schiff-based sensor capable of detecting Cu+2 and Al+3 using two different modes10.
DISCUSSION
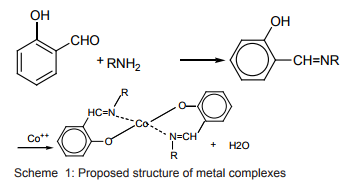
Bidentate Schiff Base Metal complexes New Schiff bases of salicylaldehyde and their Cobalt(II) derivatives Scheme (1) have been prepared and tested for their antitumor activities. Several of these, particularly the cobalt derivatives, have shown significant Inhibitory action against mouse cancers11.
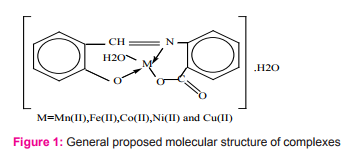
The complexes of Mn(II) and Ni(II) with Schiff base derived from salicylaldehyde and 2-amino benzoic acid have been prepared and characterized as a neutral complex with 1:1 metal to ligand ratio12Figure (1).
Yousif at el prepared and characterized a new metal complex derivatives of 2N-salicylidene-5-(p-nitro phenyl)- 1,3,4-thiadiazole, HL Scheme (2) with the metal ions Vo(II), Co(II), Rh(III), Pd(II) and Au(III). The complexes obtained are monomeric with square planar geometry except VO(II) and Co complexes which existed as a square pyramidal and tetrahedral geometry respectively. The preliminary in vitro antibacterial screening activity revealed that complexes 1–5 Figure (2) showed moderate activity against tested bacterial strains and slightly higher compared to the ligand, HL13.

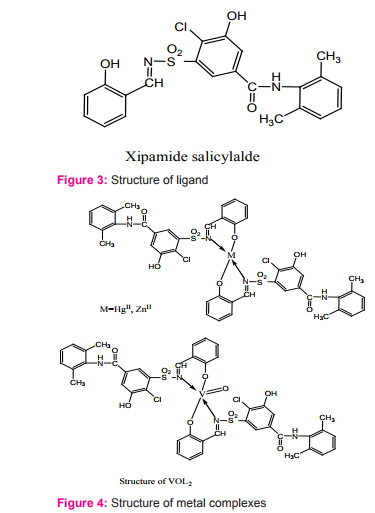
Malik and coworker reported the synthesis and characterization of metal complexes of Schiff base derived from xipamide, a diuretic drug. The bidentate ligand is derived from the inserted condensation of 5-aminosulfonyl- 4-chloro-N-2,6-dimethylphenyl-2-hydroxybenzamid (Xipamide) with salicylaldehyde in a 1:1 molar ratio Figure (3). Using this bidentate ligand, complexes of Hg(II), Zn(II), and VO(IV) with general formula ML2 have been synthesized Figure (4). All the complexes are nonelectrolytic in nature with 1:2 [M:L] ratio. Complexes of Hg(II) and Zn(II) have tetrahedral geometry via deprotonated phenolic oxygen and azomethine nitrogen atoms and VO(IV) complex have square pyramidal geometry. The pure drug, synthesized ligand, and metal complexes were screened for their antifungal activities against Aspergillus niger and Aspergillus flavus. The ligand and its Hg(II) and VO(IV) complexes were screened for their diuretic activity too.The complexes are found to have higher biological activities as compared to the respective ligand and the parent drug14.
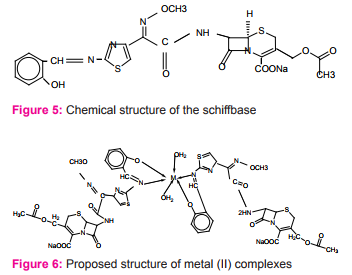
New complexes of Schiff base ligand (derived from cefotaxime with salicylaldehyde with transition metals) prepared Figure (5) and characterized as nonelectrolyte complexes [ML2 (H2 O)2 ] with an octahedral geometry for Co(II), Ni(II), and Zn(II) complexes while a tetragonal geometry for Cu(II) complex Figure (6). All complexes were tested for invitro antibacterial activity against some pathogenic bacterial strains, namely Escherichia coli, Klebsiella pneumoniae, Pseudomonasaeruginosa, Bacillus subtilis, and Staphylococcus aureus. The results show that the metal complexes possess superior antibacterial activity than the Schiff base which could be used for the development of novel antimicrobial materials15.
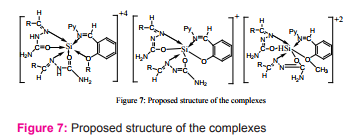
Silicon(IV) complexes containing mixed ligands: Shiffbases (AH) derived from 2- or 3- amino-pyridine with 2-hydroxy- or 3-methoxy- or 2-hydroxy-3-methoxy-benzaldehyde and benzaldehyde semicarbazone (BSCH), have been prepared. Benzaldehyde semicarbazone acts as bidentate chelating ligand, octahedral Complexes of the type [Si(BSCH)2 (AH)]Cl4 , [Si(BSC)2 (An H)]Cl2 and [Si(BSC)2 (A)]Cl (where n=2 or 3, A=deprotonated Schiff-base ligands , BSC= dep- rotonated semicarbazone) have been proposed in neutral and basic medium, respectively16Figure (7).
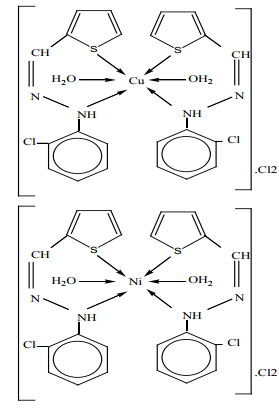
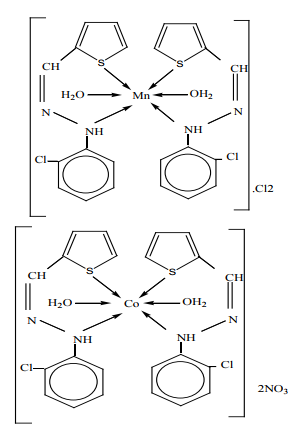
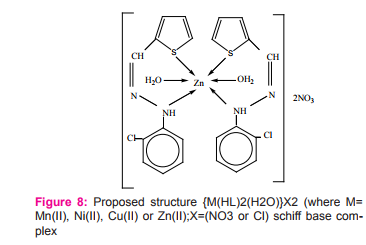
Metal chelates, [M(HL)2 (H2 O)2 ]X2 (where M= Mn(II), Co(II), Cu(II), Ni(II) or Zn(II), X= NO3 –or Cl– and HL= Schiff base moiety), have been prepared and characterized as 1:2 (metal-ligand). The coordination to the central metal atom have been through the nitrogen of the 2- chlorophenyl hydrazine (–Ph–NH-) group and the sulfur atom of the thiophene ring forming distorted octahedral complexes Figure (8). The Schiff base and its metal chelates have been screened for their in vitro antibacterial activity against four bacteria, gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) and two strains of fungus (Aspergillus flavus and Candida albicans). The metal chelates were shown to possess more antibacterial activity than the free Schiff-base chelate 17.
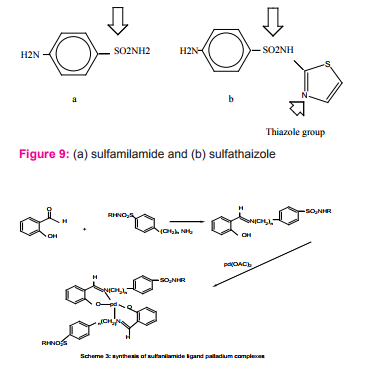
Robin R. and coworkers prepared and characterized Schiff bases complexes derived from sulfanilamides or aminobenzothiazoles Figure (9) with Pd(OAc)2 as complexes of the type PdL2 Scheme (3,4). Palladium complexes and Schiff bases have been investigated as antifungal agents against Aspergillus niger and Aspergillus flavus18.
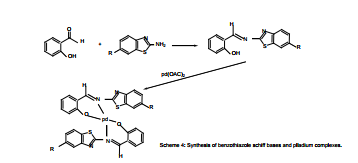
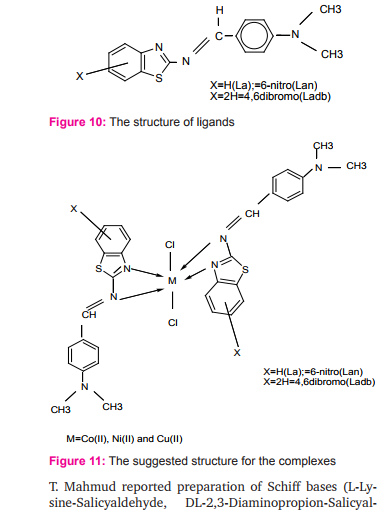
Cobalt(II), nickel(II) and copper(II) with Schiff bases derived from (2-aminobenzothiazole,6-nitro- 2aminobenzothiazole,4,6-dibromo-2-aminobenzothiazole) and 4-N dimethyl bezaldehyde to give ligands (La, Lan and Ladb) were prepared in 1:2 ratio (metal: ligand) Figure (10). These measurements indicated that the ligands coordinate with metal(II) ion in a bidentate manner through the nitrogen atoms in ligands with general formula [ML2Cl2] Where M=Co (II), Ni (II) and Cu (II), ( L=La, Lan, or Ladb) Figure (11)., Octahedral structures were suggested for metal complexes19.
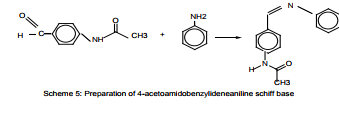
T. Mahmud reported preparation of Schiff bases (L-Lysine-Salicyaldehyde, DL-2,3-Diaminopropion-Salicyaldehyde and 4-acetylamido benzylidene aniline) in basic media (using 2M NaOH) Scheme (5). A copper(II) complex with 2,2′-bipyridine and L-lysine was synthesized and characterized as a monomer with distorted square planar geometry, or a distorted octahedron if the long axial coordination from the perchlorate ions is admitted. The asymmetric unit contains two formula units of [Cu (L-Lysine) (2,2′-bipyridine)] with two perchlorate ions and one water molecule5 .
Tridentate Schiff Base Metal complexes
Mounika and coworkers reported a new Schiff base, 3-ethoxy salicylidene amino benzoic acid (ETSAN) Scheme (6), has been synthesized from 3-ethoxy salicylaldehyde and 2-amino benzoic acid. The ligand act as neutral and tridentate coordinating through nitrogen atom of the azomethine and oxygen atoms of hydroxyl group of the 3-ethoxy salicylaldehyde beside the hydroxyl group of the carboxyl group of the 2-amino benzoic acid respectively. All complexes are non-electrolytes and show 1:1 metal: ligand ratio with octahedral geometry Figure (12). Biological studies of these complexes reveal that they show better activity when compared to that of the ligand20.

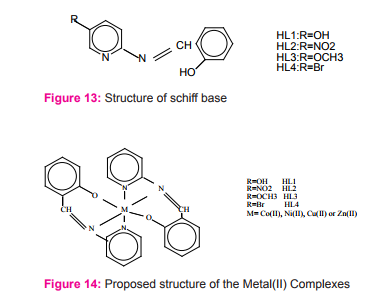
Novel transition metal [Co(II), Cu(II), Ni(II) and Zn(II)] complexes of substituted pyridine Schiff-bases Figure (13) have been prepared and characterized with general formula [M(L)2] where [M=Co(II), Cu(II), Ni(II) and Zn(II) and HL=HL1 , HL2 , HL3 and HL4 ] and an octahedral geometry. The synthesized Schiff-bases act as deprotonated tridentate with Co(II), Ni(II) and Zn(II) ions Figure (14). The Schiff bases and their complexes have been screened for antibacterial activity against the strains such as Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. The complexed Schiff bases have shown to be more antibacterial against one more bacterial species as compared to uncomplexed Schiff-bases21.
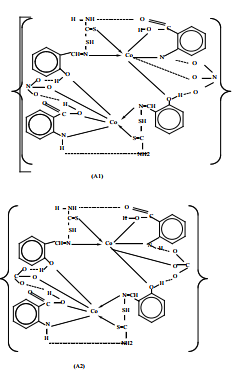
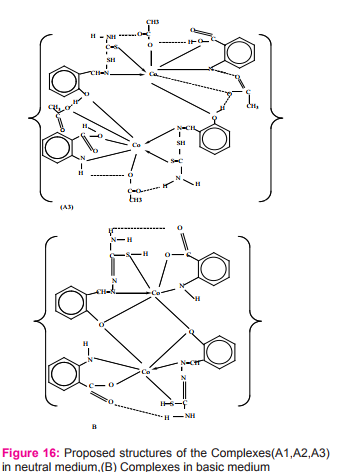
Z. F. Dawood and M. W. Ibrahim reported Preparation and characterization of new cobalt (II) complexes with mixed ligands including salicylaldehyde thiosemicarbazone-STH2 and carboxylic acid-AH2 {salicylic acidSH2 or anthranilic acid-AnH2 or phthalic acid-PH2 } Figure (15) as [Co2 (AH2 )2 (STH2 )2 (NO3 )2 ](NO3 )2 or [Co2 (AH2 )2 (STH2 )2 Xn] in neutral medium whereas, in basic medium as [Co2 (AH)2 (STH)2 ] {where X = CO3 2- or CH3 CO2- , n = 2 or 4} with octahedral geometries forming dimer (binuclear complexes)22Figure (16).

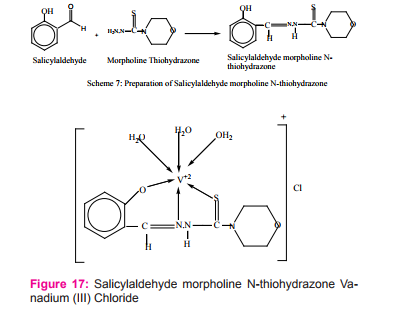
Saxena synthesized and characterized metal complexes of Ti (III), V (III), VO (IV), CO (II) and MN (III) with salicylaldehyde and thiohydrazones ligand Scheme (7) and proposed an octahedral geometry for all the synthesized complexes23Figure (17).
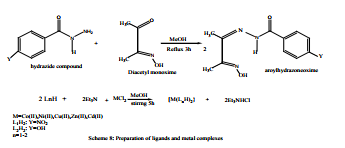
Mohammed M. Al-Ne’aimi at el have been prepared Mononuclear complexes of two novel ligands {Diacetylmonoxime-4- nitrobenzoylhydrazone (L1 H2 ), Diacetylmonoxime-4- hydroxybenzoylhydrazone (L2 H2 )} Scheme (8) with [Co(II), Ni(II), Cu(II), Zn(II) and Cd(II)] as [M(Ln H)2 ] where (n =1,2) in the presence of Et3 N. The metal complexes [M(Ln H)2 ] are proposed to be six-coordinated with a N4 O2 donor environment through the oxime nitrogen, the imine nitrogen and the enolic oxygen atoms while the phenolic hydroxyl and oxime hydroxyl groups of aroylhydrazonemonoxime moiety do not participate in coordination24.
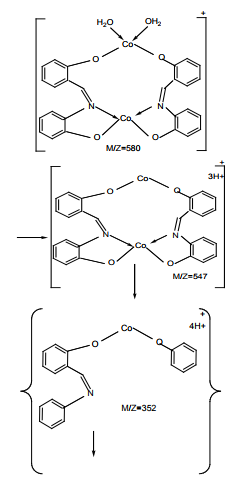
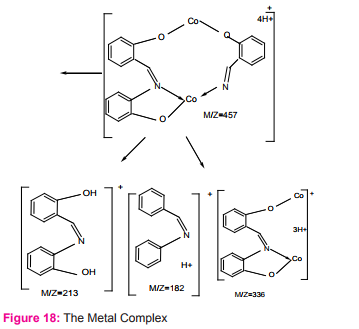
Dinuclear complexes from salicylaldehyde and 2-aminophenol with Cu (II), Ni (II) and Co (II) Figure (18) were obtained by new synthetic route and characterized. Low temperature intramolecular ferromagnetism was exhibited by homodinuclear complex while the heterodinuclear complexes showed antiferromagnetic coupling25.
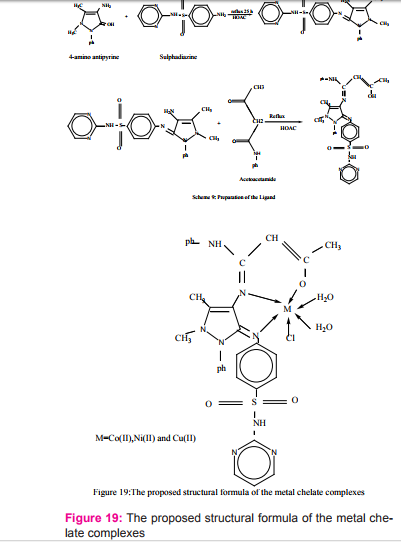
New Schiff base ligand derived from 4-Amino antipyrine, sulphadiazine and acetoacetanilide were prepared Scheme (9) and reacted with metal salts in 1:1 ratio (metal: ligand). The complexes have the general formula [MLCI.2H2 O] where M = Co(II), Ni(II) and Cu(II) Figur (19). The ligand coordinate with the metal (II) ion in neutral tridentate manner through the azomethane nitrogen atoms, and oxygen group of the acetoacetanilide forming octahedral complexes26.
Tetra dentate Schiff Base Metal complexes
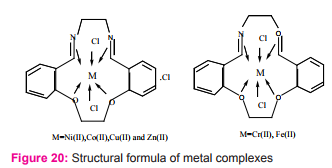
Mostafa M. and coworker synthesized macro-cyclic Schiff base ligand resulted from thecondensation of {bisaldehyde and ethylenediamine was prepared (7, 8, 15, 16, 17, 18-hexahydrodibenzo (a, g) (14) annulene)} (L) and its complexes were synthesized and characterized as 1:1 [ML] complex with octahedral structure for the all complexes via (N2 O2 ) group act as a tetradentate ligand and two chlorides as monodentate ligands Figure (20). They reported using of neutral chelating ligand as selective reagent to determine iron (III) in different types of natural water within recovery test and described that the metal complexes are more potent/ antibacterial than the parent Schiff base ligand against one or more bacterial species27.
Salicylaldehyde 2-chlorobenzoyl hydrazone (H2 LASSBio-466), salicylaldehyde 4-chlorobenzoyl hydrazone (H2 LASSBio-1064) Figure (21) and their complexes [Zn(LASSBio466)H2 O]2 and [Zn(HLASSBio-1064)Cl] were evaluated in animal models of peripheral and central nociception, and acute inflammation. All studied compounds significantly inhibited acetic acid-induced writhing response. All compounds showed levels of inhibition of zymosan-induced peritonitis comparable or superior to indomethacin, indicating an expressive antiinflammatory profile28.

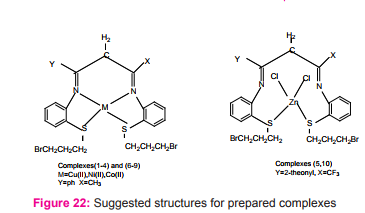
Two new tetra dentate ligands (L1 and L2) N,N’-bis{(oaminophenylthio)bromo propyl} 1-phenyl butane 1,3-dilidene (L1), N, N’-bis (3- bromo propyl (phenylthio) imino)1,1,1-trifluoro -3-(2-theonyl) acetone (L2) were prepared Scheme (10) and reacted with M= Co(II), Ni(II), Cu(II) and Zn(II) chloride salts to give [M(L)]Cl2 and [Zn(L)Cl2 ] Figure (22) as tetrahedral complexes via nitrogen atoms of the azomthine and two sulfur atoms while for zinc (II) complexes from the two sulfur atoms and two chloride ions29.

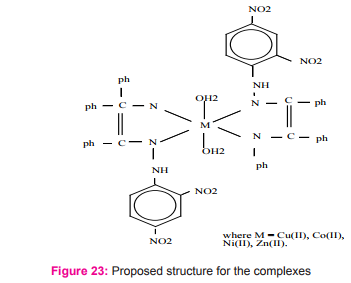
New Schiff base chelates of Cu(II), Co(II), Ni(II) and Zn(II) derived from benzil-2,4-dinitrophenylhydrazone with aniline have been synthesized Figure (23) and characterized to suggest tentative structures for the complexes30.
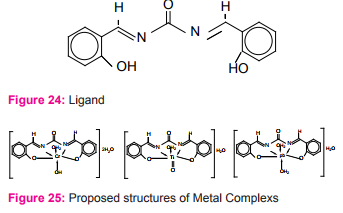
Three new metal complexes of Cr(III), Pb(II)) and TiO(IV) ions with a Schiff base derived from salicylaldehyde and urea Figure (24) have been investigated with 1:1 [M:L] ratio. The coordination behavior of the metal ions towards to the investigated Schiff base takes place through –C=N and –OH groups31 Figure (25).
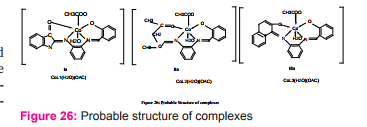
Tetra dentate N2 O2 type complexes of Co(II) have been synthesized by the condensation of o-phenylenediamine, salicylaldehyde and isatin/ naphthaldehyde/acetyl acetone and characterized as [ML(H2 O)(OAc)] with octahedral geometry Figure (26). The metal complexes have been screened for their antibacterial and antifungal activity. DNA cleavage activities of Schiff bases and their metal complexes were monitored by agarose gel electrophoresis method in the presence of H2 O2 32.
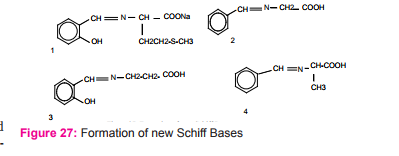
N. Kumar at el reported preparation of Schiff bases (Salicyladehyde glycine, DL-2,3-Diaminopropion-Salicyaldehyde, benzylidene glycine and 4-acetylamido benzylidene aniline) in basic media (using 2M NaOH) figure (27) and described the applications of shiff bases and their copper(II) complexes as antimicrobial activities, antifungal activities, antiviral activities. Tetra dentate Schiff base and its metal complexes with Mn (II), Ni (II), Cu (II), and Zn (II) show miscellaneous effect on membrane in amylose production33.
CONCLUSION
Salicyaldehyde and their derivatives can be good chelating ligands when they condensed with amines in 1:1 and 2:1 ratio to form bi, tri and tetra dentate Schiff base ligands suitable to form complexes with Metal ions. These Metal-Schiff base complexes have been shown to exhibit a broad range of biological activities, including antifungal, antibacterial, antimalarial, anti-proliferative, antiinflammatory, antiviral, and antipyretic properties.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors /publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Dakin, H.D.Catechol. Org. Synth. 1923; 3(28).
2. Horning, E.C.H., M. G.; Dimmig D. A.3-Carbethoxycoumarin. Org. Synth. 1948;28(24).
3. Cleiton M. da Silva, D.L.d.S., Luzia V. Modolo, Rosemeire B. Alves, Maria A. de Resende, Cleide V.B. Martins, Angelo de Fatima Schiff bases: A short review of their antimicrobial activities. Journal of Advanced Research 2011; 2: 1-8.
4. Rishu Katwal, H.K.a.B.K.K. Applications of Copper – Schiff’s Base Complexes: A Review.Sci. Revs. Chem. Commun. 2013; 3(1):1-15.
5. Mahmud T.Synthesis and characterization of the amino acid Schiff bases and their complexes with copper(II). in School Of Chemistry2010; University Of Manchester: Manchester:p 4,5.
6. Bader, N.R.Applications of Schiff’s Bases Chelates in Quantitative Analysis A Review.Rasayan J. Chem. 2010;3(4): p. 660-70.
7. Zoubi, W.A.Biological Activities of Schiff Bases and Their Complexes: A Review of Recent Works.International Journal of Organic Chemistry 2013;3: 73-95.
8. Anant Prakash, D.A. Application of Schiff bases and their metal complexes-A Review. International Journal of ChemTech Research Oct-Dec 2011;3(4):1891-96.
9. Pallavi Goel, D.K., Sulekh ChandraSchiff’s Base Ligands and Their Transition Metal Complexes as Antimicrobial Agents. J. Chem. Bio. Phy. Sci. Sec. A 2014; 4(3):1946-64.
10. Gou, Q., Wu, Wang, Luo, and Liu A highly selective chemosensor for Cu+2 and Al+3 in two different ways based on Salicylaldehyde Schiff. Inorganic Chemistry Communications 2011;14(10).
11. Ernest M. Bodneti, W.W. Schiff Bases of Salicylaldehyde and Their Cobalt(ll) Derivatives as Antitumor Agents. Stillwater. Proc. Of The Okla. Acad. of Sci, 1965:107-11.
12. Ado, H.N.A.a.I. Studies of Mn (II) and Ni (II) complexes with Schiff base derived from 2-amino benzoic acid and salicylaldehyde. Biokemistri March 2011;23(1):9-16.
13. Emad Yousif , A.M., Khulood Al-Sammarrae, Nadia Salih, Jumat Salimon, Bashar AbdullahMetal complexes of Schiff base: Preparation, characterization and antibacterial activity. Arabian Journal of Chemistry 2013:1-5.
14. Suman Malik, S.G., Bharti Jain, Archana Singh and Mamta Bhattacharya Synthesis, Characterization, and Biological Evaluation of Some 3d-Metal Complexes of Schiff Base Derived from Xipamide Drug. International Journal of Inorganic Chemistry 2013:1-6.
15. Aurora Reiss, M.C.C., Emilia Amzoiu and Cezar Ionuu Spî- nuTransition Metal(II) Complexes with Cefotaxime-Derived Schiff Base: Synthesis, Characterization and Antimicrobial Studies. Bioinorganic Chemistry and Applications:1-17
16. Z. F.Dawood, M.A.A.-S.Preparation and Characterization of Some Silicon (IV) Complexes. Sciences & Technologie Juin. 2008; A(27): 49-54.
17. Omar B. Ibrahim, M.A.M., Moamen S. RefatNano Sized Schiff Base Complexes with Mn(II), Co(II), Cu(II), Ni(II) and Zn(II) Metals: Synthesis, Spectroscopic and Medicinal Studies. Canadian Chemical Transactions 2014;2(2):108-12.
18. Robin R. Coombs, M.K.R., Johanna M. Blacquiere, Joshua C. Smith, J. Scott Neilsen, Yoon-Seo Uh, et al.Palladium(II) Schiff base complexes derived from sulfanilamides and aminobenzothiazoles. Transition Metal Chemistry2005; 30: 411–18.
19. Saleh A. Ahmed, A.O.M., Adnan A. Humada, Ihmood K. AL-juboori, Emad M. OsajSynthesis and Characterization of Some Schiff Bases(derived from thiazole)and Their Complexes With Co(II),Ni(II) and Cu(II). Journal of Kirkuk University –Scientific Studies2009; 4(2): p. 37-45.
20. K. Mounika, B.A., J. Pragathi, and C. Gyanakumari Synthesis¸ Characterization and Biological Activity of a Schiff Base Derived from 3-Ethoxy Salicylaldehyde and 2-Amino Benzoic acid and its Transition Metal Complexes. J. Sci. Res. 2010;2(3): 513-24.
21. Zahid H. Chohan, A.M.a.C.T.S. Transition Metal Ion Complexes Of Schiff-Bases. Synthesis, Characterization And Antibacterial Properties. Metal Based Drugs 2001;8(3):137-43.
22. Z. F. Dawood, M.W. Ibrahim Preparation and Characterization of Some Cobalt (II) Complexes Containing Mixed Ligands (Salicylaldehyde Thiosemicarbazone and Carboxylic Acids).National Journal Of Chemistry 2006; 21: 24-31.
23. Saxena A.Synthesis and characterization of schiff base salicylaldehyde and thiohydrazones and its metal complexes. Advances in Applied Science Research 2013; 4(4): 152-54.
24. Mohammed M. Al-Ne’aimi, M.M.A.-K.a.S.J.M. Synthesis and Characterization of Co(II) Ni(II) Cu(II) Zn(II) and Cd(II) Complexes with Aroylhydrazonemonoximes.Raf. J. Sci. 2012;23(4):51-69.
25. VD Bhatt, S.R. Preparation and Properties of Dinuclear Schiff Base Complexes from Salicylaldehyde and 2-Aminophenol Complexes of Cu (II) Co (II) and Ni(II). Chemical Sciences Journal 2012;2012.
26. Layla A. Mohammed, A.J.K., Nadia H. AubaidSynthesis and Characterization of New Schiff Base Ligand with Its Some Complexes Derived from 4-Amino Antipyrine, Sulphadiazine and Acetoacetanilide. Acta Chim. Pharm. Indica 2013;3(2):111-18.
27. Mostafa M. H. Khalil, E.H.I., Gehad G. Mohamed, Ehab M. Zayed, Ahmed BadrSynthesis and characterization of a novel schiff base metal complexes and their application in determination of iron in different types of natural water. Open Journal of Inorganic Chemistry 2012; 2:13-21.
28. Walfrido Bispo Junior, M.S.A.-M., Marina A. Alves, Anayive Perez-Rebolledo, Gabrieli L. Parrilha, Eduardo E. Castellano, et al.Analgesic and Anti-Inflammatory Activities of Salicylaldehyde 2-Chlorobenzoyl Hydrazone (H2LASSBio-466), Salicylaldehyde 4-Chlorobenzoyl Hydrazone (H2LASSBio-1064) and Their Zinc(II) Complexes. Molecules 2011;16.
29. N. H. Buttrus, C.L., M. Refat Synthesis and characterization of some new mononuclear complexes of Co(II), Ni(II), Cu(II) and Zn(II) with N2S2 donor Schiff base ligands. International Journal of Enhanced Research in Science Technology & Engineering April 2014; 3(4):156-60.
30. N. Raman, S.R., C.ThangarajaCopper(II) cobalt(II) nickel(II) and zinc(II) complexes of Schiff base derived from benzil-2,4 dinitrophenylhydrazone with aniline. J. Chem. Sci. July 2004;116(4): 215–19.
31. F.A. Abdlseed, M.M.E. Preparation and spectroscopic investigation of a Schiff base metal complexes. International Journal of PharmTech Research 2009;1(4):1097-103.
32. A. Nagajothi, A.K., S. Chitra and K. Parameswari Synthesis and Characterization of Tetradentate Co(II) Schiff Base Complexes : Antimicrobial & DNA Cleavage Studies. International Journal of Research in Pharmaceutical and Biomedical Sciences Oct – Dec 2012;3(4):1768-78.
33. Navneet Kumar, P.S., Astha pareekSynthesis of New SchiffBase Complexes and Their Applications. International Journal of Applied Research & Studies Feb 2013;2(2):1-6.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License