IJCRR - 13(22), November, 2021
Pages: 73-79
Date of Publication: 20-Nov-2021
Print Article
Download XML Download PDF
Study of Incidence of Acute Kidney Injury in Acute Myocardial Infarction and its Impact on Hospital Outcome
Author: Deshpande A, Shingade P, Deshpande S
Category: Healthcare
Abstract:Introduction: Acute kidney injury(AKI) is a common complication after acute myocardial infarction (AMI), affecting 10 to 55% of the patients.1-4 The mechanisms causing AKI in the first few days after an AMI are multifactorial, including systemic and renal hemodynamic changes secondary to an impaired cardiac output and an imbalance of vasodilators and vasoconstrictors, the use of contrast media, and immunological and inflammatory kidney damage resulting from crosstalk between the heart and the kidney Material and Methods: The study was conducted on 224 ST-segment elevations myocardial infarction (STEMI) patients admit�ted to a tertiary care hospital during December 2018-December 2020. The patients were divided into two groups depending on the development of AKI according to KDIGO (Kidney disease improving global outcomes) guidelines. Results: Out of 224 patients, 57 patients (25.45%) developed AKI and 167 patients (74.55%) did not develop AKI. So, the incidence in our study was 25.45%. When various risk factors were compared in both the group's diabetes and higher BMI (body mass index) were found to be significantly associated with AKI. The mortality in the AKI group was 28.07% while in the non-AKI it was 1.79%. In-hospital complications like cardiogenic shock left ventricular failure (LVF), arrhythmias were associated with increased incidence of AKI. Using multiple logistic regression analysis, age, presence of diabetes, tachycardia on admission, raised blood sugar on admission, decreased ejection fraction (EF), presence of cardiogenic shock and arrhythmia were independent risk factors for AKI. Conclusion: AKI in STEMI was associated with increased mortality and complications.
Keywords: Acute kidney injury (AKI), ST-segment elevation myocardial infarction (STEMI), Cardiogenic shock
Full Text:
INTRODUCTION-
Ischemic heart disease is the leading cause of death among adults in developed as well as in developing countries and accounts for a substantial fraction of the total disease burden globally. AKI (Acute kidney injury) is a common complication after acute myocardial infarction (AMI), affecting from 10 to 55% of the patients.1-4
AKI in STEMI has been consistently associated with a worse outcome, and namely with strikingly higher short-term and long-term mortality rates.3,5,6,
The overall survival of patients with ST-elevation myocardial infarction (STEMI) has significantly improved during the past two decades, due to the combined use of novel pharmacologic therapies and aggressive revascularization strategies.7 The interest of cardiologists is now shifting towards subsets of patients whose mortality remains very high, thus contributing to the overall mortality of STEMI. Those developing AKI represent a critical example of STEMI patients associated with a poor prognosis. A growing amount of data confirms the clinical and prognostic relevance of AKI in this clinical setting. However, it is worth noting that the current guidelines on the management of STEMI patients do not focus much attention on AKI. Surprisingly, while recommendations exist for the management of rare STEMI-associated complications, such as mitral valve rupture or Dressler pericarditis, no clear indications are provided on the management of this frequent complication.6,7Indeed, The National Confidential Enquiry into Patient Outcome and Death (NCEPOD) Adding Insult to Injury AKI Study reported, in 2009, that only 50% of patients who died with AKI in different clinical contexts received good care. Therefore, this study was carried out to know the incidence of AKI in acute MI and its impact on hospital outcomes.
MATERIAL AND METHODS-
This prospective observational study was carried out on 224 patients of ST-elevation myocardial infarction (STEMI) in the intensive care unit of the tertiary care centre from December 2018-December 2020. Patients with acute ST-segment elevation myocardial infarction proven by ECG (ST-segment elevation > 0.1mV in at least 2 contiguous leads) and cardiac enzymes (Positive Troponin I or CPK-MB) were included in our study. Patients who presented with Non-ST Elevation MI (NSTEMI) /Unstable angina (UA), who had evidence of chronic kidney disease, who were on renal replacement therapy and any prior use of nephrotoxic drugs were excluded from the study. A complete history including sociodemographic characters and risk factors was taken. Detailed clinical examination and relevant investigations were done. Two groups were made according to the development of AKI based on KDIGO guidelines. Various complications and outcomes were compared between the two groups.
Statistical analysis- Collected data were entered into a Microsoft Excel spreadsheet. Tables and Charts were generated using Microsoft word and excel software. Continuous variables were presented as Mean ±SD. Categorical variables were expressed in frequency and percentages. Continuous variables were compared between with and without AKI by performing an independent t-test. Categorical variables were compared by performing a chi-square test. For small numbers, Fisher exact test was used. Multiple logistic regression analysis was performed to identify independent risk factors of acute kidney injury in patients of MI. Adjusted Odds ratio, 95%confidence interval & p-values were reported. P-value < 0.05 was considered as statistical significance. Statistical software STATA version 14.0 was used for data analysis.
RESULTS-
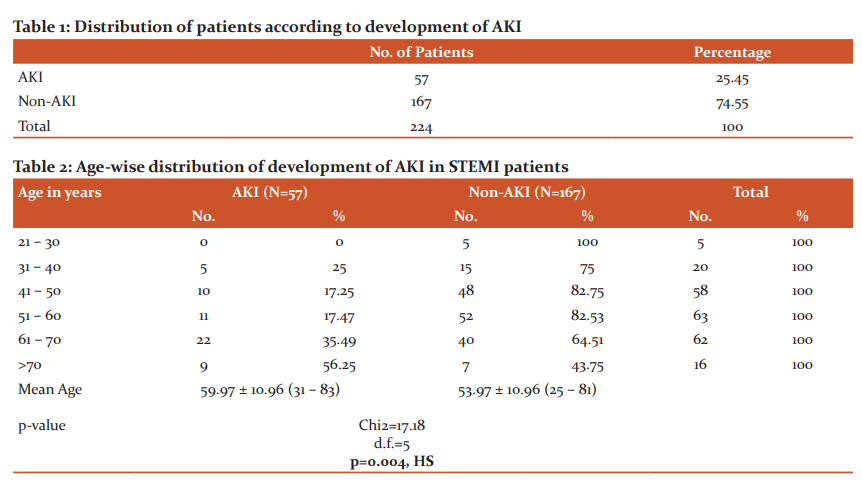
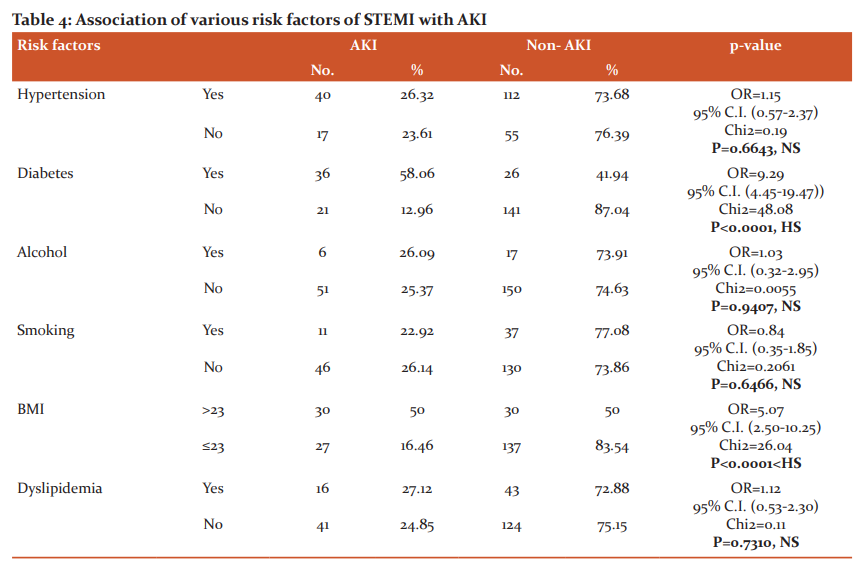
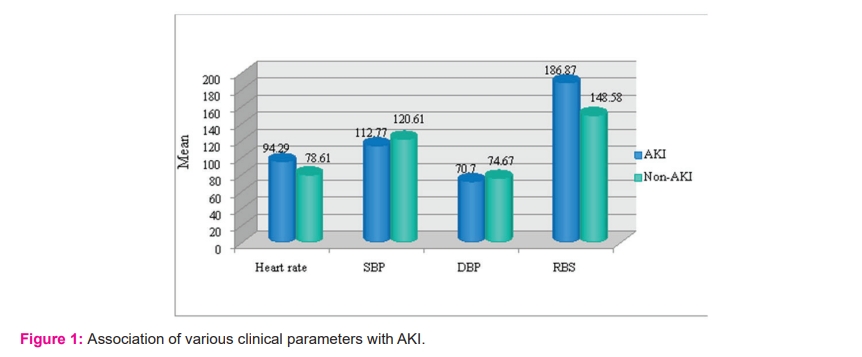
Out of 224 patients, 57 patients (25.45%) developed AKI and 167 patients (74.55%) did not develop AKI(Table-1). So, the incidence in our study was 25.45%. The mean age of patients in the AKI Group and Non-AKI Group was 59.97 ±10.96 and 53.97±10.96 respectively(Table-2). There was a statistically significant difference between mean age and incidence of AKI. Out of 224 patients, 137 were males (61.16%) and 87 were females (38.84%). Out of the total of 137 males, 41 males (29.93%) developed AKI. Out of the total of 87 females, 16 females (18.40%) developed AKI(Table-3). There was no significant difference between the two groups. When various risk factors were compared in both the groups, diabetes and higher BMI were found to be significantly associated with AKI. Alcohol, smoking, hypertension and dyslipidemia were not found to be significant(Table-4). The maximum number of patients is 41.9% who had AWMI (anterior wall myocardial infarction). There was no statistically significant difference in the area of myocardium involved and AKI. Various clinical parameters at the time of admission were compared between the two groups. Heart rate, systolic blood pressure, diastolic blood pressure and random blood sugar were found to be highly significant(Figure-1). Mean EF in the AKI group was found to be 38.85±6.65 and in the non-AKI group, it was 44.04±5.29(Table-5). This indicates that lower EF is significantly associated with the development of AKI. Patients of AKI were divided based on severity(Figure-2). Out of 57 patients, 23 patients were in mild, 22 is moderate and 12 in severe category respectively. Total 44 patients (19.64%) developed cardiogenic shock. 33 patients (75%) developed AKI and 11 patients (25%) did not develop AKI(Table-6). There was a statistically significant increase in the number of patients developing AKI who developed cardiogenic shock.
A total of 29 patients (12.94%) had evidence of LVF in the study. Out of the 29 patients, 25 patients (86.20%) developed AKI and 4 patients (13.80%) did not develop AKI. There was a statistically significant difference in both groups.
A total of 18 patients (8.03%) in the study developed arrhythmias. 15 patients (83.33%) developed AKI and 3 patients (16.67%) did not develop AKI. This difference was found to be statistically significant.
Total 7 patients (3.12%) in the study developed AV blocks. 1 patient (14.29%) developed AKI and 6 patients (85.71%) did not develop AKI. Statistically, there was no significant association of AV blocks with AKI.
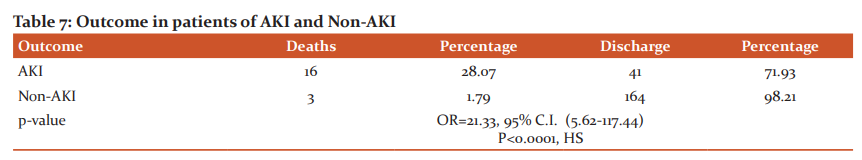
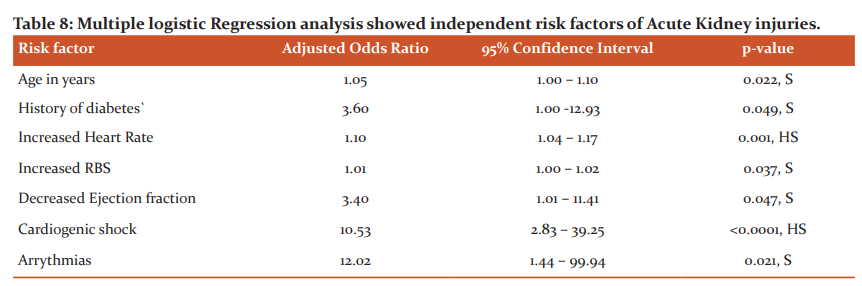
Total 19 deaths (8.48%) occurred in the study. 16 deaths (28.07%) occurred in AKI and 3 deaths (1.79%) occurred in non-AKI(Table-7). This difference was statistically highly significant. It means that mortality was very high in patients who had AKI. A higher length of hospital stay was seen in deaths and discharge of the AKI group. Using multiple logistic regression age, history of diabetes, tachycardia, hyperglycemia, low EF, presence of cardiogenic shock and arrhythmias were independent risk factors for AKI (Table-8).
DISCUSSION-
In our study out of the total 224, 57 developed AKI (25.45%) and 167 patients did not develop AKI (74.55%). The incidence of AKI in our study was 25.45%. The overall incidence of AKI is reported 10 to 55 % in various studies.1-4 Wang et al. and Amit Amin et al. reported incidence of 26% and 22.5% respectively which was similar to the present study.8,9
The mean age in the AKI group was 59.97±10.96 and in the non-AKI group was 53.97±10.96 respectively. When compared statistically the difference was found to be significant. Rodrigues et al., Reinstadler et al. and Bruetto et al. reported similar findings.1,10,11 So higher the age higher the chance of developing AKI. The reason could be that in the absence of a specific disease, the kidney undergoes age-dependent structural and functional alterations leading to a significant decrease in renal mass, functioning nephron numbers, and baseline kidney function.12
Out of the total 224 patients of STEMI, there were 137 males (61.16%) and 87 females (38.84%).
Other studies like Yan Bei Sun et al. and Bruetto et al. also showed male preponderance.1,13
Various risk factors were compared between both the groups which included hypertension, diabetes, alcohol intake, smoking, BMI and dyslipidemia. Out of this diabetes and increased BMI were significantly associated with AKI.
Reinstadler et al. and Moriyama et al. also studied same factors. But the statistically significant difference was found only with diabetes.11,14 Various clinical and laboratory parameters on admission were considered which included heart rate(HR), systolic blood pressure(SBP), diastolic blood pressure(DBP), random blood sugar(RBS). So, tachycardia, low SBP, low DBP and increased RBS were found to be significantly associated with AKI.
Tachycardia was also found significant by Moriyama et al. and Fox et al.14,15
Increased heart rate could be explained by increased sympathetic activity in left ventricular failure and reactive tachycardia due to cardiogenic shock. Low SBP and DBP were found highly significant in the studies by Moriyama et al. and Wang et al.9,14
This is explained by the fact that reduction in blood pressure which could be a result of cardiogenic shock reduces renal perfusion thus leading to AKI.In our study stress hyperglycemia was associated with increased incidence of AKI. This fact was also supported by Yan Bei Sun et al.
Hyperglycemia may thus represent an epiphenomenon of the stress response mediated by cortisol and catecholamines, whose release is elicited by the hemodynamic compromise or myocardial damage. Hyperglycemia exerts a direct negative impact on renal function.16
When LV function was assessed by echocardiography there was a significant association of low EF with AKI. Reinstadler et al. showed a significant association of cardiac magnetic resonance (CMR) determined cardiac function and myocardial damage with the development of AKI.
Out of 224 patients, 44 patients (19.64%) had evidence of cardiogenic shock.
Out of 44 patients, 33 patients had AKI (75%) and 11 patients (25%) did not develop AKI. There was a significant association between cardiogenic shock and the development of AKI.
Cong Wang et al. reported 36 patients with cardiogenic shock. Out of which 32 patients (88.88%) had AKI and 4 patients (11.12%) did not have AKI. This indicates that the presence of cardiogenic shock increases the susceptibility to AKI. When LVF was considered in our study, 29 patients (12.94%) had LVF.Out of 29 patients, 25 patients (86.20%) developed AKI and 4 patients (13.80%) did not develop AKI. So, it shows that LVF plays a major role in the development of AKI. In the study by Cong Wang et al., 76.51% of patients with LVF developed AKI.So, their findings are comparable with our study.9
There was evidence of arrhythmia in 18 patients (8.03%) out of 224.
Out of them, 15 patients (83.33%) developed AKI and 3 patients (16.67%) did not develop AKI. So, the presence of arrhythmia makes the patient prone to the development of AKI.
The finding in our study was also supported by Cong Wang et al.9
The mortality in our study was 28.07% in the AKI group and 1.79% in the non-AKI group. So, the development of AKI in STEMI increases mortality. This observation was also supported by the study of Moriyama et al.14
CONCLUSION-
AKI developing in patients with acute myocardial infarction is associated with a higher rate of complications and in-hospital mortality.
Acknowledgment-authors acknowledge the immense help received from the scholars whose articles are cited and included in the references of this manuscript. The authors are also grateful to the authors and publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
CONFLICT OF INTEREST-None
SOURCE OF FUNDING-None
ETHICAL CLEARANCE- Taken



References:
1. Bruetto RG, Rodrigues FB, Torres US, Otaviano AP, Zanetta DMT, et al. Renal Function at Hospital Admission and Mortality Due to Acute Kidney Injury after Myocardial Infarction. 2012;PLoS ONE 7(4): e35496.
2. Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, et al. In-hospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. 2005;150:330–337.
3. Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168:987–995.
4. Marenzi G, Assanelli E, Campodonico J, De Metrio M, Lauri G, et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med. 2008;38:438–444.
5. Anzai A, Anzai T, Naito K, Kaneko H, Mano Y, Jo Y, Nagatomo Y, Maekawa Y, Kawamura A, Yoshikawa T, Ogawa S. Prognostic significance of acute kidney injury after reperfused ST-elevation myocardial infarction: synergistic acceleration of renal dysfunction and left ventricular remodeling. J Cardiac Fail. 2010 May 1;16(5):381-389.
6. James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al; Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409-416.
7. Stewart JA. Adding insult to injury: care of patients with acute kidney injury. Br J Hosp Med. (Lond) 2009;70:372-373.
8. Amin AP, Salisbury AC, McCullough PA, Gosch K, Spertus JA, Venkitachalam L, et al. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med. 2012;172:246-253
9. Cong Wang, Yuan-Yuan Pei. Risk factors for acute kidney injury in patients with acute myocardial infarction. Chinese Med J. 2019;132(14):1660-1665.
10. Rodrigues FB, Bruetto RG, Torres US, Otaviano AP, Zanetta DM, Burdmann EA. Incidence and mortality of acute kidney injury after myocardial infarction: a comparison between KDIGO and RIFLE criteria. PloS one. 2013 Jul 23;8(7):e69998.
11. Sebastian Johannes Reinstadler, Andreas Kronbichler. Acute kidney injury is associated with microvascular myocardial damage following myocardial infarction. Kid Int. 2017;92:743–750.
12. Rosner MH. Acute kidney injury in the elderly. Aging Health. 2009;5:635–647.
13. Sun YB, Liu BC, Zou Y, Pan JR, Tao Y, Yang M. Risk factors of acute kidney injury after acute myocardial infarction. Renal Fail. 2016 Oct 20;38(9):1353-1358.
14. N.Moriyama. Early development of acute kidney injury is an independent predictor of in-hospital mortality in patients with acute myocardial infarction. J Card. 2017;69:79–83.
15. Caroline S. Fox Shor-Term Outcomes of Acute Myocardial Infarction in Patients With Acute Kidney Injury. Circulation. 2012;125:497-504.
16. Persson PB, Patzak A: Renal hemodynamic alterations in contrast medium-induced nephropathy and the benefit of hydration. Nephrol Dial Transp. 2005;20(suppl 1):i2–i5.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License