IJCRR - 13(22), November, 2021
Pages: 17-22
Date of Publication: 20-Nov-2021
Print Article
Download XML Download PDF
Knowledge, Attitude and Practices of Informed Consent Documentation Among Researchers in Medical Colleges in Tamil Nadu - a Questionnaire-Based Study
Author: Mohesh Glad, Ramalingam Latha, Prakash Revinselvan
Category: Healthcare
Abstract:Introduction: Considering the rise in the number of health research activities amid the ongoing coronavirus disease-2019 pandemic, there is an immense need to understand the knowledge, attitude and practices of the medical researchers on the informed consent documentation process in their research studies. Academic research in medical colleges is booming for two exclusive reasons, namely, the need for research papers for academic promotions, and the student projects guided by the faculty. Objective: To determine the knowledge, attitude and practices about the Informed consent documentation (ICD) process among researchers in medical colleges. Methods: This was a cross-sectional survey done using an online questionnaire among 241 researchers in various medical colleges for their knowledge, attitude and practice on the process of ICD in their research. Results: About 91.5% of them were aware of the importance of informed consent, 85.9% believe that the ICD process safeguards the rights of the research participants and nearly 92.5% practised obtaining informed consent from the participants in their projects. Statistically significant results (p< 0.05) was found in certain intricate practices of ICD among senior faculties when compared against the junior faculties. However, the association between designation and knowledge is not much evident in this study. Conclusion: Researchers are aware of the importance of ICD in all aspects however there is a good segment of them who need training and guidance on the process of ICD to achieve complete compliance of ICD at all levels of researchers in medical colleges.
Keywords: Informed consent, Ethics, Medical research, Human participants, Research documentation
Full Text:
Introduction:
Medical research involving human participants has increased greatly in most of developing countries to improve the quality of healthcare in them, like in India.1 Researcher bear the responsibility for revealing the complete information about the research to their prospective study participants to keep them informed about the proposed research, procedures involved, goals and even the potential risk along with the benefits. International ethical guidelines consistently impose researchers to follow some kind of informed, voluntary and competent informed consent documentation to minimize the chance of exploitation of the study subjects.2
As human participants were involved in medical research, to maintain the participant's autonomy and rights, and also for the research to be guided by fundamental ethical principles to ensure the protection of their welfare, it is mandated by various national and international regulatory bodies to insist upon the collection and maintenance of informed consent documentation.3,4
Informed consent documentation, among all the other ethical practices in research, has gained the most attention among researchers. It may be because the practice of informed consent before any research had become the fundamental element that serves to protect both research participants and the researcher from any prosecution.5 Informed consent documentation is consistently required to ensure that research is informed, and voluntary and there is no exploitation of the study subjects.2
The Indian council of medical research, the primary authority in India that regulates medical research published revised new guidelines on ethics in the year 2018. This revision has happened after a decade and hence it becomes a necessity for every researcher to get introduced to the new guidelines. Changes in the informed consent documentation (ICD) process was evident in the recent guidelines and to understand if the researchers are aware of these changes, we conducted the present study to ascertain the knowledge, attitude and practices about the ICD among researchers in medical colleges.
Materials and methods
This research was conducted after obtaining approval from the Institutional Ethics Committee (SSSMCRI/IEC/2019/500) and written informed consent was obtained from each of the study participants. The choice of the decision on participation in the online survey was inducted within the online survey form and only those who have consented got the opportunity to access the remaining part of the questionnaire of survey.
This cross-sectional descriptive study was conducted with an online questionnaire which was emailed to researchers (300) in different medical colleges across the state of Tamilnadu and the responses were collected online (google forms). The total number of samples was estimated as 241, based on an earlier publication,5 with the desired confidence interval of 95% and an expected drop-out of 10%. Both male & female faculties working in the medical colleges (Professors, Associate professors, Assistant Professors and Tutors, who have completed at least 1 year of service as Assistant professors and with at least one completed research work and one publication were included in the study. Tutors, Assistants with less than 1 year of experience and those without any publication were excluded from the study.
A standardized questionnaire with 30 questions on knowledge, attitude and practice (KAP) was designed in Google forms - a free survey tool provided by google was used to collect the data. All the questions were excerpts from the regulations for informed consent documentation (ICD) in Indian Council of Medical Research (ICMR) guidelines 2018. Both the face and content validation of the questionnaire was done before the initiation of the study. The questionnaire had a brief introductory note and a statement on the confidentiality of the data collected to the respondents.
The study questionnaire was emailed to most of the researchers in the state through email and their responses were populated in the Google response sheet. Multiple response options were blocked and hence one participant submitted one response only. These responses were collected between May 2019 to December 2019. Responses were then transferred to an MS office-Excel sheet and were coded to perform the required statistical analysis. Percentage distribution of the medical researchers in the various sections of the study objectives was analysed for statistical significance and is described in the results of the study. Descriptive statistics were used to explain the demographic data. A Chi-square test was done using SPSS v 19.0 to find if there is any association between the designation of the subjects and their KAP about the informed consent documentation process. Statistical significance was set for a value of p <0.05.
Results
The study included 241 participants from various medical colleges across Tamil Nadu. Out of the selected study participants, 153 were males and 88 were females. As the study was focused on medical research, the questionnaire was distributed among Professors (30.3%), Associate Professors (17%), Assistant Professors (22.4%) and Tutors (17%), who were involved in research across all disciplines in medical colleges of Tamil Nadu. 51.9% of the selected population had a minimum of 1 to 5 publications and 24.5 % of them had more than 10 publications. Though 94.6% of the participants had a registered Institutional Ethics committee in their working Institution, only 79.7 % had undergone some training in Ethics.
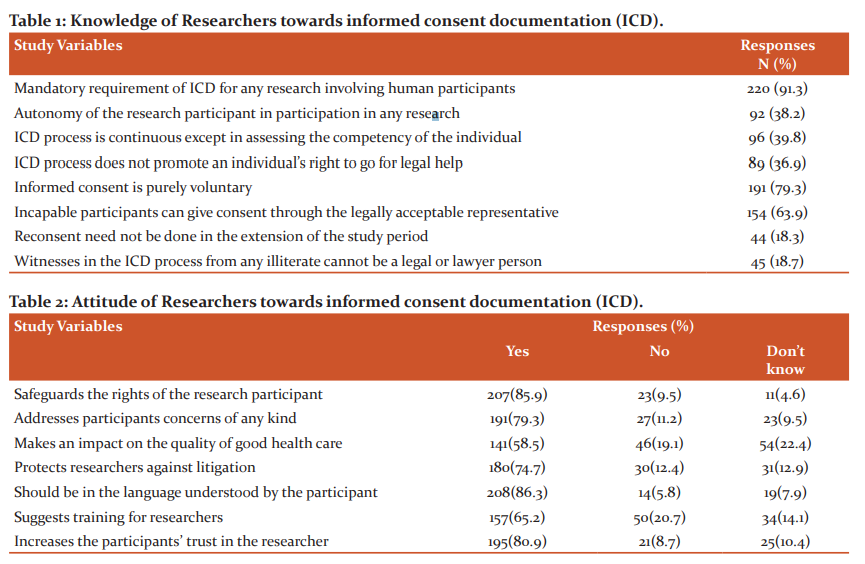
Knowledge about Informed consent documentation (ICD)
The results obtained from the participants on their existing knowledge regarding the rights of the study participants, procedures and requisites for obtaining ICD are shown in table 1. On average, 37.5% of the right answers were obtained from the participants. The remaining 62.5% of answers chosen by the participants were incorrect which lead to a significant value (fig1).
Only 47 of the total participants were aware of the fact that the studies involving biological hazards are not to be exempted from any informed consent waiver. 23.7 % of them were aware of the use of community consent which is applicable in situations when individual consent cannot be obtained as it will change the behavior of the individual. Forty-nine percent of participants agreed that the prospective study subjects should not be intimidated while obtaining informed consent. The importance of obtaining informed assent and written informed consent from children between 12 to 18 years of age is less understood by 35 % of participants as shown in figure 2.
Attitude towards Informed consent documentation (ICD):
When the respondents were questioned about their opinions on the method of obtaining consent, 23.7% indicated that it was a complicated process. An equal amount of participants accepted it to be a mandatory process as well (29.5%). In comparison, only 60.6% are mindful of the need for informed and written approval from ICD. as shown in fig 3. Table 2 displays the participants' responses to questions regarding the attitude towards obtaining informed consent for research involving human subjects. More than 65% of positive responses have been recorded for the questions on the importance of maintaining ICD. < 10% of the participants are yet unaware of the advantages of maintaining ICD. 14.1% of participants have expressed their opinion of the requirement of training for the process to be more accurate and proper.
Practices followed in Informed consent documentation (ICD):
Table 3 demonstrates the existing practices of ICD by the research participants in their research studies.
Nearly 93% of participants report that they regularly maintain ICD in their research studies. 31.5% of participants were unaware or do not follow the procedure of giving a copy of ICD to their research participants. Only 57.3% of participants have reported that they regularly obtain informed consent before the start of the study. Others have been doing it during the study or after that just for documentation purposes. 33.2% of participants store the obtained ICD and the data for up to 5 years. 24.5% of participants have reported that they do not store the data after the completion of the study.
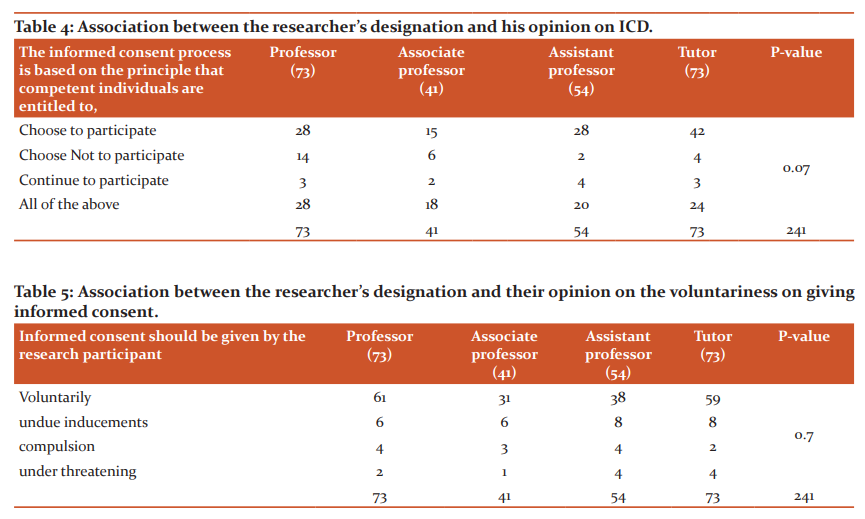
Association between the participant’s designation and the KAP
Table 4 and Table 5 demonstrate the fact that there is no association between the designation of the researcher and the information on the informed consent documentation process that they have acquired as evident due to the absence of any statistically significant differences among the groups. However, 189/241 (78.4%) of the research participants correctly emphasized the voluntariness of the informed consent. Similarly, another non-significant association was seen in the item that was asking for the informed consent process. Nearly 66% of the faculty correctly declared the purpose of the informed consent in the participant's perspective was either to participate or to quit the research.
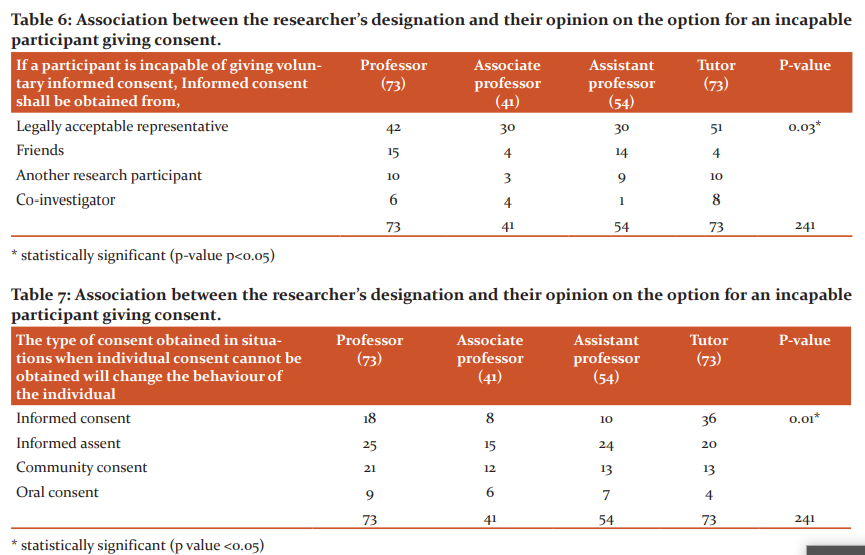
Table 6 shows a statistically significant association between the designation of the study subjects and the right person to obtain consent if the participant is incapable of giving informed consent (p-value 0.03).
Table 7 demonstrates a highly statistically significant association between the designation of the study participants and their awareness of community consent ( p<0.01).
Discussion:
Our study was unique in conducting an assessment after the rollout of the revised guidelines on Ethics by the Indian council of medical research in the year 2018. The questionnaire served by itself as a tool to sensitize the participants about the revised and recently modified guidelines. The response rate was acceptable (80.33%) and the results showed the standards of the medical researchers across the state concerning their knowledge, attitude and practices of Informed consent documentation in their research.
Knowledge aspects of ICD:
The study participants were from medical colleges across the state of Tamil Nadu in India. All the participants were medical college affiliates and about 1/3rd of them were women. There was a significant gap in the knowledge of obtaining Informed consent and its documentation as per ICMR guidelines, 2018. The quality of research depends on the participant’s understanding of the processes involved in giving consent. Voluntary participation, processes involved in obtaining consent, and confidentiality of data obtained are some of the elements to be necessarily known and followed by the researchers involved in Medical Research.6 There was also a substantial lack of information among the researchers about the requirements for obtaining consent from children. Before giving permission, all children have the right to get the necessary information in their comprehensible language.7 The revised medical curriculum ie., the Competency-based undergraduate curriculum and the importance of the AETCOM module introduced by the Medical Council of India from the year 2019-20 might inculcate the knowledge of ethics in clinical practice and research. It involves the evaluation component also which assures the learning of bioethics at a very early part of the career.8 The need for re-consent and the role of a legally accepted representative in informed consent documentation is also less understood by the researchers. There is an existing need for central agencies like ICMR to enhance the sensitization programs (preferably online with certification) and also the institutional ethics committees to ensure that their dynamic medical researchers are aware of the existing as well as the recently revised guidelines of the ICMR.
Attitude aspects of ICD:
Though the majority of the participants opine the process of ICD as a simple and responsible one, about one-fourth of the participants felt it to be a difficult or painstaking process. Nijhawan et al have reported that this might be due to language barriers, religious and cultural influences, patients’ false perceptions about the process and fear.9 Our study participants have demonstrated a constructive attitude about the need to obtain and retain informed consent for research purposes. Shared decision-making and explaining to patients about their concerns for participation with documentary pieces of evidence, especially concerning the risks and benefits involved in the research could be some of the strategies that can be adopted for increasing the validity and reliability during Informed consent documentation.10,11 They also acknowledged the point that the autonomy and anonymity of the patient or participant is always the central point of medical ethics.12 Majority of the participants have suggested pieces of training on ICD processes, which is possible that every registered Institutional ethics committee can organize and conduct annual review meetings or sensitization programs on the ICD process.
Practice aspects of ICD:
While most participants documented their practice of recording informed consent during their study, only half of them did the procedure before collecting data from their research subjects. The data collected after the end of the study was never retained by one-fourth of the participants, and it was only 33.2 percent who have been able to retain it for 5 years. The participants are unaware of the need for data protection in support of any legal proceedings arising from their research study. They were also not familiarized with the participant’s right to receive a copy of the informed written consent form. Educating the medical researchers with the revised guidelines and helping them imbibe good ICD processes in their research studies would bring out a healthy and safe research environment and better research in the entire nation.
Association between the designation of the researcher and the informed status on ICD.
Medical research involves different types of research designs and when human participants have involved in the informed consent documentation process as per the guidelines issued by the ICMR will be followed. Hence the knowledge about informed consent documentation is very much mandatory for any explorer in medical research. However, a change in designation happens over years of academic experience with 1-2 publications. Hence the association between knowledge of the ICD process and the designation is meagre. With sensitization programs run by the ICMR on the new ethical guidelines and the researcher experiencing himself with the guidelines and the reinforcing committees to implement strictly the ICD process, it becomes an easy process to train the junior researchers on this knowledge, practice or attitude.
Limitations:
This study was conducted among medical researchers in the southernmost state of India. The prevalence and practices of ICD in other parts of the country should be explored for more valuable information. Data were collected using an anonymous questionnaire using google forms and hence the opinions are very qualitative yet subjective. ICD practices among other healthcare sectors also should be explored to find the extent of ICD practices and the reasons for any setbacks.
Conclusion:
This is a unique study exploring the KAP on the informed consent documentation process by medical researchers using human participants. The knowledge about ICD was better, yet provides scope for updating the researchers with the revised guidelines on ethics by ICMR. The positive attitude on the importance of ICD is promising as this paves way for further sensitization and empowerment of the researchers with information on the revised guidelines. Their practices on ICD was good, however, the legal aspects of the ICD and the need for re-consent are some areas that require updates. We conclude here that the fact that ICD process is appreciably being carried out by the medical researchers in this part of the country, yet, they are in dire need of pieces of training on improvising themselves with the revised guidelines of the regulatory body.
Conflict of Interest: The authors declare that there is no conflict of interest of any kind.
Source of Funding: Nil
Acknowledgements: Authors hereby acknowledge the support rendered by Dean, Shri Sathya Sai Medical College & RI. We also thank Prof.Dr.Saurabh Shrivastava, Department of Community medicine for helping us in fine-tuning the manuscript. The authors also thank the faculties who volunteered themselves as study participants in this study.
Authors contribution :
Mahesh G and Selvan R have participated in the conception and design, or approval of the final version. Mahesh G and Ramalingam L have participated in the analysis and interpretation of data.
Mohesh G and Ramalingam L have contributed to drafting the article or revising it critically for important intellectual content.
.png)
References:
1. Normile D. The promise and pitfalls of clinical trials overseas. Science 2008; 322 (5899): 214-6.
2. Sayed, Nancy E. Kass. Attitudes of Sudanese researchers on obtaining informed consent from study subjects involved in health research. Sudan. J. Public Health 2007; 2(2): 95-102.
3. World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Jahrbuch Für Wissenschaft Und Ethik. 2009;14(1):233-8.
4. Macrae DJ. The Council for International Organizations and Medical Sciences (CIOMS) guidelines on ethics of clinical trials. Proceedings of the American thoracic society 2007;4(2):176-9.
5. Sinha RK, Hiba I. Knowledge, attitude and practice of informed consent. Management in Health 2016; 20(1):14-21.
6. Del Carmen MG, Joffe S. Informed consent for medical treatment and research: a review. The oncologist 2005;10(8):636-41.
7. Levy MD, Larcher V, Kurz R. Informed consent/assent in children. Statement of the Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP). Eur J Pediatr 2003;162(9):629-33.
8. Chandrashekar J, Seby JG. Knowledge, attitudes and practices related to healthcare ethics among medical and dental postgraduate students in south India. Indian J. Med. Ethics 2014;11(2):99-104.
9. Nijhawan LP, Janodia MD, Muddukrishna BS, Bhat KM, Bairy KL, Udupa N, Musmade PB. Informed consent: Issues and challenges. Adv Pharm Technol Res2013;4(3):134.
10. Grady C. Enduring and emerging challenges of informed consent. N Engl J Med 2015; 372(9):855-62.
11. Kegley JA. Challenges to informed consent: new developments in biomedical research and healthcare may mark the end of the traditional concept of informed consent. EMBO reports 2004; 9: 832-6.
12. Anderson OA, Wearne IM. Informed consent for elective surgery—what is best practice?. J R Soc Med 2007;100(2):97-100.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License