IJCRR - 13(16), August, 2021
Pages: 77-83
Date of Publication: 30-Aug-2021
Print Article
Download XML Download PDF
Association of Gut Microbiota with Ischemic Stroke: A Review
Author: Malhotra Jaspreet Kaur, Gupta Shruti, Kumari Hema, Kumar Kaushalendra, Kumar Gaurav
Category: Healthcare
Abstract:Introduction: The gut microbiota and central nervous system (CNS) interact in a bidirectional manner. In response to physical and psychological stressors affecting secretion, motility and immune reactivity, the CNS modulates gut functioning. However, alterations in gut microbiota may cause neurochemical and behavioural changes. Cerebral stroke generates an immune and inflammatory response in the brain and various immune organs as well as affect the gut microbiota Objective: The present review paper aims to provide a comprehensive discussion on the association of gut microbiota with ischemic stroke. Methods: NCBI-PubMed database has been extensively searched to collect information regarding the gut microbiome and ischemic stroke. Results: In this paper, we discussed the gut-brain axis, regulation of intestinal γδT Cells, gut-immune response to brain injury, effect of gut microbiota on brain development, behaviour and neurotransmitters. Further, we highlight the pathways for systemic inflammatory response syndrome, Trimethylamine N-Oxide, and T regulatory cells to understand the underlying mechanism of neuronal dysfunction. Conclusion: CNS dysfunction due to ischemic insults affects gastrointestinal function through the vagus nerve signal, immune pathways, endocrine systems and neurotransmitters. The intestinal microbiota plays an imperative role in retaining metabolic homeostasis, immune system, and protection against pathogens.
Keywords: Gut microbiota, Ischemia, Cerebral stroke, Blood-Brain barrier, Neurotransmitters, CNS
Full Text:
INTRODUCTION
Ischemia is the restriction of blood supply to the bodily tissues that causes a decrease in oxygen level thereby hampering cellular metabolism thus affecting cellular function and growth. A decrease in the availability of nutrients and inefficient removal of metabolic waste also leads to ischemia. The build-up of metabolic waste, mitochondrial damage and inability to maintain cell membranes may lead to tissue damage.1 Cerebrovascular accident (or stroke) are a type of brain injury resultant of lack of oxygen supply to a region of the brain due to lack of blood supply that could ultimately lead to permanent neurological deficits or even death.2 Based on the pathophysiology, brain stroke could be categorized into ischemic and hemorrhagic stroke. The cerebral ischemic stroke or cerebral ischemia accounts for 85% of all stroke occurrences rest being a hemorrhagic stroke. It was found out that the maximum number of stroke cases might be associated with behavioral factors like smoking, obesity, poor diet, and a sedentary lifestyle.3Recentstudies also suggest that altered gut microbiota could be considered as a risk factor for stroke. The gastrointestinal tract is considered as the major immune organ of the body which has the largest stock of immune cells accounting for about 70%.2 The human gastrointestinal tract is home to the dynamic and complex microbial population known as gut microbiota or gut microflora which has a marked effect on the host metabolism and maintenance of the healthy living condition of individuals.4 Multiple factors play a contributing role in the establishment of the human gut microflora since early childhood, diet being the fundamental factor throughout the life span. The intestinal microbiota plays an imperative role in retaining metabolic homeostasis, immune system, and protection against pathogens.5 The altered gut bacterial composition or microbial imbalance is known as dysbiosis has been found to have a direct association with the pathogenesis of many infections and diseases.6 The metabolites derived out of the gut-microbiota have a role in diseases like stroke and cardiovascular diseases.7 Trimethylamine N-oxide (TMAO) is a waste metabolite that is synthesized by gut microbiota which finds its association with both stroke and CVD.8 A vast difference occurs between the gut microbiome of an ischemic stroke patient and healthy individuals. However, there still lies a concern about the direct link between the patient’s intestinal flora and the brain stroke. A stroke dysbiosis index (SDI) differentiates between the microbial disparities in healthy individuals and patients.9 The GI tract tends to be a very unique system of the body due to its intrinsic nervous system, also known as the enteric nervous system; indicating an effect of the microbiota of neural and glial cells.10 The microbiota also tends to affect brain development and behaviour since gut microbiota plays a role in the maintenance of monoamine neurotransmitters levels.11The blood-brain barrier is responsible for proper maintenance of the functioning of neurons, a change in the gut microbiota could bring about an alteration in permeability of BBB.12 Gut microbiota holds the ability to synthesize the neurotransmitters and tend to play a mediating role in the interaction with the nervous system.13
Due to the high socioeconomic burden of ischemic stroke and its pathological consequences in enduring subjects, understanding the pathophysiology and association of ischemic stroke with dysbiosis is a major priority for neurological dysfunction research. The present review work provides a comprehensive discussion of the microbiota, gut-brain axis, regulation of intestinal γδT Cells, gut-immune response to brain injury, the effect of gut microbiota on brain development and behavior as well as on neurotransmitters.
MICROBIOTA-GUT-BRAIN AXIS
A bidirectional communication exists between the brain and the gut and its diverse microbiota which is referred to as the microbiota-gut-brain axis (Fig. 1). The signals are interchanged between the brain and the gut via both neuronal and non-neuronal mechanisms. The brain to gut signaling also called top-down signaling, the gut receives a neuronal signal via the parasympathetic and sympathetic nervous system. These neuronal signals are responsible for gut permeability, gut motility, immune cell activation, and microbiota composition.14
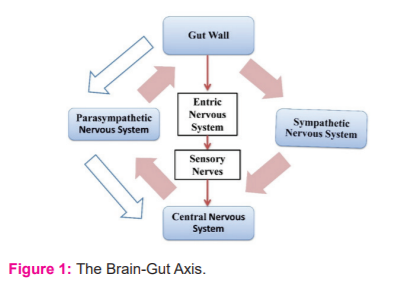
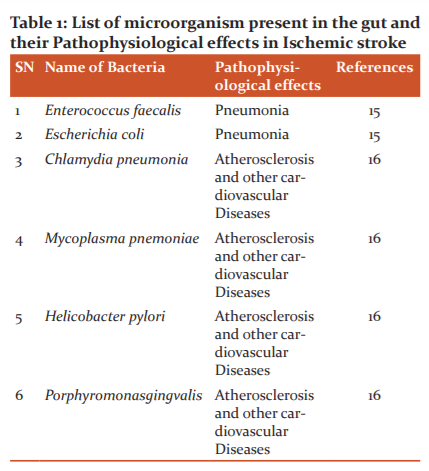
The gut to brain signaling also called bottom-up signaling occurs via several different processes. Firstly, the vagus nerve plays a dual role in transmitting signals between the gut and the brain. The vagus nerve is braced by the microbial compounds, hormones (like serotonin, peptide YY), and metabolites as illustrated in fig. 2. This results in stimulating hypothalamic neurons that are responsible for the regulation of pituitary secretions. Secondly, immunogenic endotoxins like lipopolysaccharide (LPS) from the microbiota can prompt neuroinflammation directly or via peripheral immune cell activation that can migrate to the brain. Lastly, the microbiota induced release of neurotransmitters, short-chain fatty acids, and bile compounds that enter the blood-brain barrier (BBB) through a systemic blood circulatory system.14This entire top-down and bottom-up signaling is a complex process cycle inclusive of the immune cells and neuronal signaling (fig. 2). Table 1 represents a few microorganisms that have a pathophysiological effect on the body that results in ischemic stroke. The vagus nerve, stress hormones, and the neurotransmitters are responsible to establish a communication link between the gut microbiota and the central nervous system that produces an effect on CNS thus playing a role in its functioning and leading to the problem like a stroke under certain circumstances.

BRAIN-GUT AXIS AFTER STROKE
The predominant reason for ischemic stroke in the middle cerebral occlusion which is marked by inflammation and immune response. The treatment strategy involves the following - a) Thrombolysis by intravenous administration of plasminogen activator and; b) Thrombectomy to remove blood clot physically. With a restriction of the therapeutic window of 4.5 hrs.after the onset of the stroke. It is followed by many pathological events that are triggered by brain ischemia after recanalization and reperfusion, so-termed as ischemia-reperfusion injury. The two major problems are the prolongation of the therapeutic window of thrombolysis and endovascular thrombectomy and the development of treatment of tissue ischemia-reperfusion injury; which when solved would act as an effective treatment of ischemic stroke.17
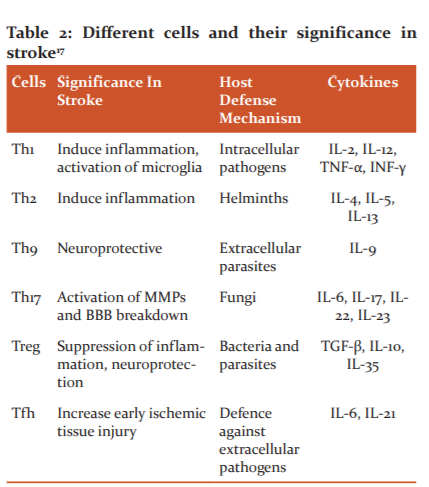
The gut microbiome and the immune system has said to be coevolved and the interaction among them is required to keep animals and humans healthy. Dysbiosis generally leads to disparity in the T-cell population that triggers several autoimmune and inflammatory disorders. Th1 cells secrete pro-inflammatory cytokines IL-2, IL-12, TNF-α, and INF-γ to promote cellular immune response making it involved in the pathogenesis of stroke.18 Th-2 promotes a humoral response against allergens and parasites. The IL-17 produced by γδ T-cells doesn't require antigen to produce an immune response thus it is more rapid.17 The γδT-cells are abundant in the gut from where they cluster at the leptomeninges structure of the brain post-stroke.19An absence of Treg cells increases the post-stroke activation of the inflammatory cells including T-cells and microglia which ultimately indicates Treg cells play an evident role in dampening post-ischemic inflammation.20 Treg smothers the differentiation of Th17 and proliferation of γδT-cells in the gut so to maintain an anti-inflammatory environment. Various cells and their significance in the stroke are illustrated in Table 2.17
The gastrointestinal tract is a very unique system of the body due to the presence of its intrinsic nervous system, referred to as the enteric nervous system (ENS); which is made of an outer myenteric plexus and the inner submucosal plexus. The ENS plays an important role in gastrointestinal motility, adsorption, fluid secretion, and blood flow.10 A study showed that in rodents enteric neurons are largely formed during embryogenesis and in early postnatal life. During which, a minute population of Sox10-expressing neural crest-derived cells colonize the foregut and ultimately undergo multiplication to colonize the entire bowel thus forming to glial cells and neurons.21,22 A major percentage of the body's serotonin is produced by enterochromaffin cells (EC), a type of enteroendocrine cells located in the gut epithelium. It has been recorded that gut microbiota can induce the secretion of serotonin in the gut.23 The fact that mucosal and neuronal serotonin are different pools that are supported by different forms of rate-limiting enzymes tryptophan hydroxylase, which is used by neuronal and non-neuronal cells, with tryptophan hydroxylase (TPH) 2 used by neurons while TPH1 is used by EC cells.24 The activation of serotonin in ENS produces a neurogenerative and neuroprotective action.
Systematic inflammatory response syndrome after stroke
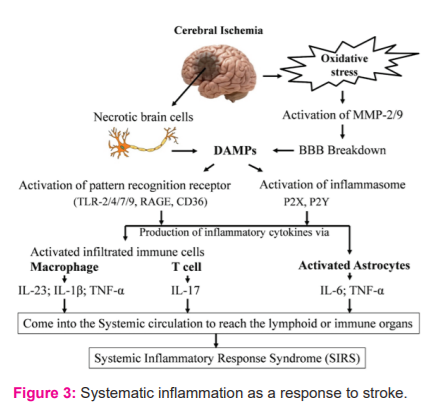
Damaged tissue after the stroke release inflammatory molecules and cellular debris, called the Damage-associated molecular patterns (DAMPs). These DAMPs hold a crucial role in the initiation of an inflammatory and immune response through pattern-recognition receptors (PRRs) like Toll-like receptors (TLR). This type of immune response is also called sterile inflammation.17 DAMPs along with cytokines are produced by brain ischemic tissues are released in circulation to reach the lymphoid or immune organs. This is a severe form resulting in systemic inflammatory response syndrome (SIRS). DAMP molecules come into the systemic circulation through the broken BBB and initiate SIRS after the stroke. This is accompanied by leaky gut post-stroke. This marks the initiation of systemic inflammation that begins with the innate immune response (mediated by innate immune cells neutrophils, microglia, macrophages and innate lymphocytes like γδT cells), to which follows the activation of adaptive immune cells after the stroke (mediated by T and B lymphocytes) 25 26as explained in fig. 3
GUT-IMMUNE RESPONSE TO BRAIN INJURY
The gastrointestinal tract is constituted by a wide range of pathogenic and commensal microorganisms. The intestinal lumen commensal flora, the mucosal immune system, and the epithelial immune system together constitute the GI. The GI has an efficient mucosal barrier and a multi-dimensional immune system consisting of scattered immune cells and lymphoid tissues called the gut-associated lymphoid tissues to protect pathogenic microorganisms and dietary antigens.17 27
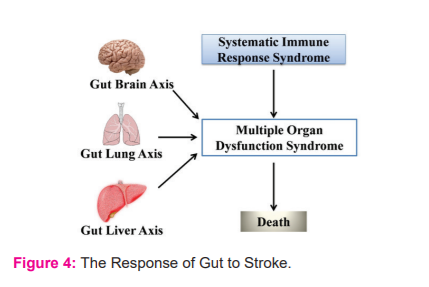
The divulge of lumen bacteria and the toxins from the intestine into the circulation due to an increase in permeability or any injury, which is called the leaky-gut or sepsis depending on the severity; leads to systemic inflammation and thus serves as systemic inflammation response syndrome (SIRS).17 SIRS along with gut-lung, gut-liver, and gut-brain axis can result in multiple organ dysfunction syndromes (MODS) that could ultimately lead to death as explained in fig. 4. To restrict the invasion of toxins and gut microorganisms during the transport of nutrients, the gut has about 70% immune cells of the entire immune system along with the largest population of macrophages.17 Thus explaining how stroke finds its association with long-term multiple organ dysfunction.
The blood-brain barrier (BBB) is responsible for the maintenance of the proper functioning of neurons by controlling the passage of molecules across brain parenchyma and the blood. An experiment conducted with germ-free mice, that lacks normal gut microbiota showed an association with increased permeability of the BBB. The leakage of Evans blue dye from the vessels, use of radio-labeled ligand for in vivo Positron Emission Tomography, and damage of neurons after administration of R4A antibody confirmed this relation between increased permeability and in the lack of gut microbiota. The increased permeability of the BBB in germ-free adult mice could be because of disorganized tight junctions and reduced expression of transmembrane proteins claudin-5 and occludin. Tight junctions play a determinant role in the functional perpetuation of the BBB. The germ-free mice had a lesser amount of occludin and claudin-5 along with cytoskeletal changes and redistribution of tight junction protein altered the BBB integrity. Reduced expression of both occludin and claudin-5 is associated with increased permeability of BBB. The dispensing of normal flora from the pathogen-free mice increased the expression of occludin and claudin-5 that marked the decreased permeability of BBB. These observations direct towards the fact that the expression of occludin by the brain endothelial cells is sensitive towards the intestinal gut microbiota and its changes.12Humans show marked changes in their gut microbiota during the first and the third trimester of the pregnancy.28 Also, there is the permeability of embryonic BBB to maternal antibody.12 All these observations lead to an inference about the contribution of maternal gut microbiota to increased nutritional demand during the later stages of pregnancy that would require demanding control of BBB of the growing progeny.
Role of Regulatory T-cells in ischemic stroke
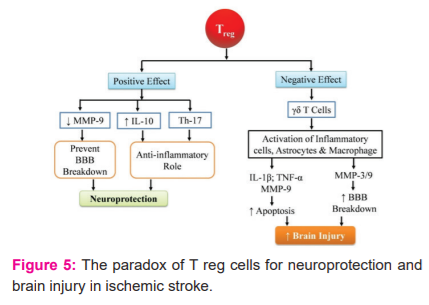
Treg cells are the type of lymphocytes finding their significance in immune homeostasis, suppression of extra immune response, and maintaining the immunological self-tolerance.29 A loss of Treg cells is considered to be the main cause of autoimmune disorders.30, 32The Treg cells can induce cytolysis of T cells in a granzyme-mediated manner and can also inhibit the T effector cell function through negative signals. Studies suggest that the number of circulating Treg cell fall drastically in the second day after the stroke onset, which is followed by an increase on the 7th day which sustains till the 3rd week.29 Treg cells also tend to cause an addition to the ischemic stroke via microvascular dysfunction and also tend to compromise the neurological function recovery. Thus influencing the stroke and its outcomes and also modulating the immune system.29 Inflammation is the major element in the pathophysiology of cerebral ischemia. The innate and adaptive immune cells play a role in the pathophysiology of stroke and play a role in secondary neurodegeneration by the release of neurotoxic factors and reactive oxygen and nitrogen species along with exopeptidases.19The paradox of T reg cells for neuroprotection& brain injury in ischemic stroke is illustrated in the fig. 5.
The positive effects
Certain studies have demonstrated that Treg cells play a valuable role in post-stroke events by stopping the secondary infarct growth in response to ischemia.29 The Treg cells tend to assert a protective mechanism by the release of the interleukin-10 cytokine (IL-10), which is an anti-inflammatory cytokine. IL-10 has a neuroprotective role and plays a role in the reduction of brain injury which is followed by artery occlusion.29 Treg also tends to confer protection against artery occlusion by slowing the rise of metalloproteinases-9 (MMP-9).29 The stroke-induced MMP-9 production leads to brain damage due to neutrophil infiltration which leads to the breakdown of the blood-brain barrier, on the contrary, Treg adoptive treatment inhibits MMP-9 production in the brain and blood within 1 day after ischemia 29 Tregshow their benedictory effect by directly acting on the peripheral immune system rather than acting on the brain tissue directly. The intestinal Treg plays a vital role in maintaining an anti-inflammatory abode in the gut by the smothering of differentiation of the T helper 17 cells and escalating the gδTcells19.
The negative effects
Studies suggest that the Treg cells could aggravate ischemic-reperfusion damage within 24 hrs of the stroke. Experimental studies have shown an increase in neuronal damage by inducing microvascular dysfunction by the Treg cells due to increased cerebral blood flow and reduced level of fibrin in the Treg-depleted mice within 24hrs after the artery occlusion thereby indicating less tissue damage. Treg cells were accumulated in the infract and the peri-infract area but couldn't cross the blood-brain barrier and were present in cerebral vessels, thereby suggesting low chances of the Treg cells to directly interacting with the parenchymal tissue and suggesting a higher probability to disrupt the microvascular structure. The post-stroke inflammation leads to tissue regeneration and repair which could be compromised due to immunosuppression caused by Treg cells.29 A regular synergy between the epithelial microbes and the immune system leads to immune system development, maintenance, and functions. Most abundant commensal microbes are found in the intestine that acts as a powerful regulator of the lymphocyte population including regulatory T cell (Treg) and gδTcells. gδTcells are located on epithelial surfaces and are responsible to aggravate brain injury by the release of IL-17.19
Relation of Trimethylamine N-Oxide with ischemic stroke
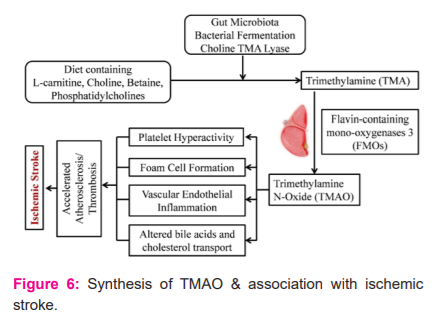
Trimethylamine N-Oxide (TMAO) is a metabolic waste product produced by gut microbiota that finds its association with stroke and cardiovascular disease. TMAO is generated through two steps. The trimethylamine (TMA) is produced from dietary nutrients like choline, L- carnitine by the gut microbiota enzyme TMA lyases. Further, in the next step, the TMA is absorbed and oxidized by hepatic flavin-containing monooxygenases (FMOs) to TMAO.8Fig. 6 shows the synthesis of TMAO and its association with ischemic stroke. The diet holds a crucial role in the formation of the TMAO. Since diet can modify the gut microbiota composition, the diet with meat, eggs, and other meat products has a high composition of phosphatidylcholine that escalates the production of TMAO. With a high-fat diet, the TMAO concentration also tends to increase in the blood.8
Few studies indicate the relation of TMAO concentration and stroke occurrence. A case-control study in a hypertensive group of the Chinese population demonstrated that a higher level of TMAO in blood marked an increased risk towards the first stroke. It was also inferred that people with low folate concentration and high TMAO concentration in blood have a higher rate of stroke. 8 31
In another case-control study on the Chinese patients with stroke and transient ischemic attack (TIA) had considerabledysbiosis of the gut microbiota, most importantly these patients showed lower plasma TMAO concentrations than control patients with asymptomatic atherosclerosis. which was explained by the fact that the treatment of stroke or TIA may have resulted in the reduction of TMAO levels.8
DISCUSSION
The gut microbiome and the immune system has said to be coevolved and the interaction among them is required to keep animals and humans healthy.The stroke-related injuries generally lead to disruption of the brain-gut axis thereby leading to the release of injury-induced DAMPs, the BBB alteration, cytokine release, leaky gut, and dysbiosis. The change in BBB after the stroke leads to increased permeability of the brain parenchyma making the entry of inflammatory and immune cells easy. The blood-brain barrier (BBB) is responsible for the maintenance of the proper functioning of neurons by controlling the passage of molecules across brain parenchyma and the blood. An experiment conducted with germ-free mice, that lacks normal gut microbiota showed an association with increased permeability of the BBB. The leakage of Evans blue dye from the vessels, use of radio-labelled ligand for in vivo Positron Emission Tomography, and damage of neurons after administration of R4A antibody confirmed this relation between increased permeability and in the lack of gut microbiota. The increased permeability of the BBB in germ-free adult mice could be because of disorganized tight junctions and reduced expression of transmembrane proteins claudin-5 and occludin. Tight junctions play a determinant role in the functional perpetuation of the BBB. The germ-free mice had a lesser amount of occludin and claudin-5 along with cytoskeletal changes and redistribution of tight junction protein altered the BBB integrity. Reduced expression of both occludin and claudin-5 is associated with increased permeability of BBB. The dispensing of normal flora from the pathogen-free mice increased the expression of occludin and claudin-5 that marked the decreased permeability of BBB. These observations direct towards the fact that the expression of occludin by the brain endothelial cells is sensitive towards the intestinal gut microbiota and its changes.12Humans show marked changes in their gut microbiota during the first and the third trimester of the pregnancy.28 Also, there is the permeability of embryonic BBB to the maternal antibodies.12 All these observations lead to an inference about the contribution of maternal gut microbiota to increased nutritional demand during the later stages of pregnancy that would require demanding control of BBB of the growing progeny.
CONCLUSION
The relationship between gut microbiota and stroke provides a promising area for research and development of control, prevention, and treatment methods for stroke. The brain-gut axis makes an important communication pathway via the vagus nerve and the neurotransmitters. This communication is essentially important for the maintenance of a healthy blood-brain barrier and also in keeping a check of behavioral activity and reducing anxiety-like responses. The role of Treg cell in ischemic stroke is also elusive that need extensive study. The exact mechanism of molecular changes during the stroke that confers a change in the brain-gut axis is still not clear. The extent to which inflammatory and immune cells change the disease progression provides a wide scope for research.
Acknowledgment: Authors are thankful to Galgotias University for providing the basic infrastructure for this work. The authors are highly grateful to Prof. Durg Vijay Rai, Former Vice-Chancellor, Shobhit University, Gangoh Saharanpur, Uttar Pradesh for the motivation and consistent encouragement during the entire review work.
Source of Funding: None
Authors’ Contribution: Malhotra Jaspreet Kaur wrote the whole manuscript; Gupta Shruti co-wrote the manuscript; Kumari Hemaand Kumar Kaushalendra co-wrote the manuscript; Kumar Gaurav provided ideas, created images and supervised the study.
Conflict of interest: None
References:
1. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2017;Dec 6;7(1):113-170.
2. Kumar G, Mukherjee S, Paliwal P, Singh S Sen, Birla H, Singh SP, et al. Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn Schmiedebergs Arch Pharmacol. 2019;Oct;392(10):1293-1309.
3. Boehme AK, Esenwa C, Elkind MSV. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017.120:472–495
4. Li N, Weng X, Sun C, Wu X, Lu M, Si Y, et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19: 191
5. Belkaid Y, Harrison OJ. Homeostatic Immunity and the Microbiota. Immunity. 2017.Apr 18; 46(4): 562–576.
6. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017.474 (11): 1823–1836
7. Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017.Mar 31;120(7):1183-1196.
8. Nam HS. Gut microbiota and ischemic stroke: The role of trimethylamine N-oxide. Stroke J. 2019.May; 21(2): 151–159
9. Xia GH, You C, Gao XX, Zeng XL, Zhu JJ, Xu KY, et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front. Neurol. 10:397. doi: 10.3389/fneur.2019.00397
10. De Vadder F, Grasset E, Holm LM, Karsenty G, Macpherson AJ, Olofsson LE, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. PNAS June 19, 2018 115 (25): 6458-6463
11. Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. PNAS February 15, 2011 108 (7) 3047-3052;
12. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014 6: 263RA158
13. Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Research. 2018.Aug 15; 1693(Pt B): 128–133.
14. Durgan DJ, Lee J, McCullough LD, Bryan RM. Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke. 2019.50:2270–2277
15. Winek K, Dirnagl U, Meisel A. Role of the Gut Microbiota in Ischemic Stroke. Neurol Int Open. 20171: E287–E293
16. Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. PNAS March 15, 2011 108 (Supplement 1) 4592-4598
17. Arya A, Hu B. Brain–gut axis after stroke. Brain Circ. 2018 Oct-Dec; 4(4): 165–173
18. Pawluk H, Wo?niak A, Grze?k G, Ko?odziejska R, Kozakiewicz M, Kopkowska E, et al. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clinical Interventions in Aging. 2020.15: 469–484.
19. Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016May;22(5):516-23.
20. Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009Feb;15(2):192-9.
21. Wang X, Chan AKK, Sham MH, Burns AJ, Chan WY. Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology. 2011Sep;141(3):992-1002.e1-6.
22. Memic F, Knoflach V, Morarach K, Sadler R, Laranjeira C, Hjerling-Leffler J, et al. Transcription and Signaling Regulators in Developing Neuronal Subtypes of Mouse and Human Enteric Nervous System. Gastroenterology. 2018Feb;154(3):624-636.
23. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015Apr 9; 161(2): 264–276.
24. Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signaling-the gut and beyond. Nature Reviews Gastroenterology and Hepatology. 2017Jul;14(7):412-420.
25. Boehme AK, Kapoor N, Albright KC, Lyerly MJ, Rawal P V., Bavarsad Shahripour R, et al. Predictors of systemic inflammatory response syndrome in ischemic stroke undergoing systemic thrombolysis with intravenous tissue plasminogen activator. J Stroke Cerebrovasc Dis. 2014Apr;23(4):e271-6.
26. Vourc’h M, Roquilly A, Asehnoune K. Trauma-induced damage-associated molecular patterns-mediated remote organ injury and immunosuppression in the acutely Ill patient. Front. Immunol. 9:1330. doi: 10.3389/fimmu.2018.01330
27. Ahluwalia B, Magnusson MK, Öhman L. Mucosal immune system of the gastrointestinal tract: maintaining a balance between the good and the bad. Scand J Gastroent. 201752:11, 1185-1193
28. Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Kling Bäckhed H, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012Aug 3; 150(3): 470–480.
29. Xu X, Li M, Jiang Y. The paradox role of regulatory T cells in ischemic stroke. Sci World J. 201310.1155/2013/174373
30. Liesz A, Hu X, Kleinschnitz C, Offner H. Functional Role of Regulatory Lymphocytes in Stroke. Stroke. 2015May; 46(5): 1422–1430.
31. Nie J, Xie L, Zhao BX, Li Y, Qiu B, Zhu F, et al. Serum trimethylamine N-oxide concentration is positively associated with the first stroke in hypertensive patients. Stroke. 2018Sep;49(9):2021-2028.
32. Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008Dec;43(2):164-74
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License