IJCRR - 13(15), August, 2021
Pages: 44-50
Date of Publication: 10-Aug-2021
Print Article
Download XML Download PDF
Therapeutic Efficacy of an Unani Polyherbal Formulation in Leucorrhoea: A Prospective Pre-and Post-treatment Clinical Study
Author: Anjum AA, Tabassum K, Sultana A
Category: Healthcare
Abstract:Introduction: Leucorrhoea or vaginal discharge is a common complaint among women of reproductive age. Reproductive tract infections originate in the lower genital tract as vaginitis or cervicitis. In conventional medicine, the available treatment is anti-bacterial and antimicrobial, which though effective, has side effects. Objective: The study was conducted to evaluate the therapeutic efficacy and safety of an Unani formulation in leucorrhoea. Methodology: This prospective, single-centre, pre-and post-treatment clinical study was conducted in patients (n=30) of abnormal vaginal discharge from the Dept. of Ilmul Qabalat Wa Amraze Niswan, National Institute of Unani Medicine, Hospital, Ben�Bengaluru. Patients received 6g powder of polyherbal formulation (Orchis latifolia L., Phaseolus mungo L., Bambusa arundinacea Retz, Portulaca oleracea L., Pistacia lentiscus L. and Boswellia serrata Roxb) twice daily after meals with water for 21 days. The outcome parameters included vaginal symptom scale (VSS) score for leucorrhoea and associated symptoms and visual analog scale (VAS) to assess low backache (LBA). Data analysis were done using paired proportion test and the Students 't' test. Results: Out of 30 patients, 25(83.3%), 4(13.3%) and 1(3.3%) showed relieved, partially relieved and no relief in the symptoms respectively. The total VSS score pre-and post-treatment was 33.03\?3.26 and 19.63\?5.47 respectively. The polyherbal formulation showed a significant difference after treatment in alleviating signs and symptoms of leucorrhoea (p < 0.001) and VAS score for LBA at each follow-up. No side effects were noted. Conclusion: The polyherbal formulation was effective in the management of leucorrhoea.
Keywords: Leucorrhoea, Unani formulation, Vaginal Symptom Scale, Visual Analogue Scale
Full Text:
INTRODUCTION:
The concept of women’s health today has become a major concern among developing countries because of the deteriorating quality of life. Reproductive Tract Infections (RTIs) is defined as the infection of the reproductive or genital tract which causes ill-health among sexually active women of reproductive age in developing countries. 1 “RTIs usually originate in the lower genital tract as vaginitis or cervicitis and may produce symptoms such as abnormal vaginal discharge, genital pain, itching and burning micturition”.2
Excessive vaginal discharge or leucorrhoea is probably physiological or pathological. During ovulation, pregnancy, emotional stress, before menstruation, use of OCP’s, sexual arousal and nutritional deficiency are included in physiological vaginal discharge that does not necessitate precise treatment, whereas, in pathological conditions like cervicitis, cervical erosion, trichomoniasis, candidiasis, gonococcus, chlamydia or bacterial vaginosis etc and secondary infection of wounds, abrasions, burns, chemical injuries and neoplasms require management.3 Prevalence of vaginal discharge (VD) in India is about 30%.4 Poor genital hygiene is responsible for the high prevalence of excessive vaginal discharge. Abnormal vaginal discharge is used when secretions from the vulvovaginal are abnormal in colour, odour, volume, or consistency.5The discharge may be thick or thin in consistency and whitish or yellowish. Among all gynaecological complaints, AVD is the commonest complaint that is associated with the psychosomatic disorder and affects the psychology of women irrespective of socio-economic status and occupation.6 It creates irritation in women’s freedom and decreases QoL.7
Vaginitis can occur with or without true inflammation of vaginal mucosa. It is observed that 50% of vaginitis is due to Bacterial Vaginosis (BV), 20-25% due to monilial infection and 15-20% due to trichomonal infection.3 Cervicitis is a state of cervical inflammation that results in an abnormal mucopurulent discharge and cervical friability.8 Cervicitis is also described as mucopurulent cervicitis in many trials.9 The sexually transmitted infections (STIs) diseases such as chlamydia, gonorrhoea, trichomoniasis, and syphilis recognized to cause cervicitis, vaginitis, urethritis and genital ulceration. Chlamydia and gonorrhoea cause grave short- and long-term complications such as ectopic pregnancy, infertility, pelvic inflammatory disease, arthritis and chronic pelvic pain. 10 The main clinical manifestation of PID is lower abdominal tenderness and abnormal vaginal discharge. The mild or subclinical PID also has the potential for damaging the reproductive health of women.11 A low threshold for diagnosis of PID is recommended to cover milder forms of PID.12
Contemporary therapy supports the use of antimicrobial that is effective but produces adverse effects in the human body. There has been a continuous increase in the recognition of persistent, recurrent and metronidazole resistant cases of reproductive tract infections, which is of serious concern. Hence, undoubtedly there is a need for alternative medicine for the treatment of abnormal vaginal discharge including protection strategies.
According to the Unani system of medicine, leucorrhoea is due to the weakness of the retentive power of the uterus.13 A weakness of this power causes accumulation of waste material in the uterus and expel in the form of abnormal vaginal discharge. In Unani medicine, the treatment for abnormal vaginal discharge includes the change in lifestyle and following hygienic conditions and management with suitable oral or local herb mineral Unani drugs. The Unani drugs are in use for ages because of their holistic approach. For the management of disease, the Unani system of medicine takes the entire constitution of the individual and lifestyle into account for diagnosing and prescribing the treatment. In the Unani system of medicine, several drugs are available for treating leucorrhoea.
Treatment goals encompass not only the amelioration of signs and symptoms of leucorrhoea but also the prevention of long-term reproductive sequelae. The Unani formulation containing Orchis latifolia L., Phaseolus mungo L., Bambusa arundinacea Retz, Portulaca oleracea L., Pistacia lentiscus L. and Boswellia serrata Roxb 13 were selected as they exhibit the properties of habis (styptic) and qabiz (astringent), muhallil auram (anti-inflammatory), and dafe taffun (anti septic), muqawwi jigar aur meda (tonic to liver and stomach), musammin Badan (nutritive) and mumsik (retentive) properties.14 Further, these drugs contain flavonoids, phenolic acids, lipids,15 volatile oils, glucosides, 16 and tannins which probably may resolve the inflammation and help in the reduction of vaginal discharge and its associated symptoms. Since it has not been validated and documented to date, therefore, an attempt was made to validate the efficacy of the aforementioned polyherbal formulation in relieving the sign and symptoms of leucorrhoea.
METHODOLOGY:
Study design, location and duration: A single centre prospective pre and post-treatment clinical study was carried out in the outpatient Dept. of Ilmul Qabalat wa Amraze Niswan, National Institute of Unani Medicine and Hospital, Bengaluru, during the year 2017-2018. The study protocol was approved by Institutional Ethical Committee, NIUM, Bengaluru under IEC No: NIUM/IEC/2016-17/015/ANQ/07 after which the clinical trial was registered in CTRI with registration number: CTRI/2018/04/013148.
Participants:
Inclusion and exclusion criteria: A total of 30 married patients between 18- 45 years of age who had abnormal vaginal discharge associated with low backache, pruritus vulvae, lower abdominal pain or dyspareunia were included. Patients with systemic and metabolic diseases, syphilis, gonorrhoea, HIV and malignancy were excluded. Patients who used contraceptives within 3 months before enrolment, pregnant and lactating women were also excluded.
Procedure:
Data collection: Patients informed consent was obtained from the patients who fulfilled the inclusion criteria. After consenting, demographic data such as age, occupation, education, socio-economic status, habitation, parity, contraceptive practice and BMI were recorded in case record form. The symptoms were recorded in the vaginal symptoms scale questionnaire. Pain assessment was done on VAS score for dyspareunia, low backache and abdominal pain. Every patient was subjected to a pelvic examination. The patient was advised to empty the bladder and then in lithotomy position vulva and perineum were examined for any discharge, erythema, excoriation, oedema etc. Cleaning of genitalia was done with 10% savlon and a Cusco’s speculum was gently introduced to note the condition of the vagina and cervix. The bimanual examination was done to assess the cervical position, consistency, cervical motion tenderness, size, shape, position, surface and tenderness of the uterus. Then the patients were subjected to routine investigations such as complete blood picture, random blood sugar and the biochemical safety profile (AST, ALT and Alkaline Phosphatase, blood urea and serum creatinine) were performed pre-and post-treatment. Specific investigations which include ultrasound pelvis to exclude pelvic pathology, VDRL and HIV I and II was done to exclude STD’s. A vaginal smear was done to diagnose the type of vaginal infection pre-and post-treatment. A Pap smear was performed to exclude cervical malignancy before treatment.
Intervention:
The polyherbal formulation included Salab Misri (Orchis latifolia Linn), Arade Moong (Phaseolus mungo Linn), Tabasheer (Bambusa arundinacea Retz), Tukhme Khurfa (Portulaca oleracea Linn), Mastagi (Pistacia lentiscus Linn) and Kundur (Boswellia serrata Roxb) in the equal quantity that was selected from the classical text.13 This formulation was selected as it was useful in leucorrhoea on the basis aforementioned Unani properties.14 Moreover, these drugs are pharmacologically proven for anti-inflammatory, anti-bacterial, anti-trichomonal activity, anti-fungal, antiseptic, analgesic, hepatoprotective and antioxidant activities. 16, 17, 18, 19,20, 21
Method of preparation and dispensing of the test drug: The medicines were provided by the pharmacy of the Institute. The drugs were identified by a pharmacognosist of FRLHT (Foundation for Revitalization of Local Health Traditions) Bengaluru. All six drugs in equal quantity were cleaned and powdered. The powder was dispatched in self-locking plastic sachets. To avoid any confusion regarding dosage, one self-locking sachet was used to dispatch. Six grams was given twice daily with water for 21 days. All patients were strictly advised to follow the instructions which were given regarding the drug dosage, diet and hygiene.
Follow-up cum assessment during and after trial: Initially at baseline vaginal discharge and its associated symptoms were thoroughly assessed by using vaginal symptoms score (VSS) and visual analogue scale for pain. Determination of vaginal pH and vaginal smear was repeated after the completion of the treatment. Patients were advised to follow-up every week during the trial and one follow up after 15 days of trial completion. During follow-up visits, the patients were assessed in the improvement of signs and symptoms using the same scales. The patients were also enquired about any adverse effects noted during the trial period. Pre- and post-treatment parameters were statistically analyzed to evaluate the efficacy of the test drug.
Assessment tools
Vaginal Symptom Scale (VSS): The VSS is a 19-question survey focusing on symptoms, self-treatment, and impact on social life, concerns about health, smell and relationship difficulties. It is available in both Spanish and English. The scoring of the scale ranges from 0-43. It is a type of questionnaire which includes symptom subscale (sum score, range 0-15), self-care subscale (sum score, range 0-4), social discomfort subscale (sum score, range 0-9), worry subscale (sum score, range 0-9) and relationship subscale (sum score, range 0-6). The grading of VSS was scored as 0-10 as no symptoms, mild score as 11-20, moderate score as 21-30 and severe score as 31-43.22
Visual Analogue Scale (VAS) for pain intensity for low backache: VAS is a methodologically sound instrument for quantitative assessment of pain. Woodforde and Merskey first reported the use of the VAS scale with the descriptor extremes “no pain at all” to “severe pain”. The VAS is self-completed by the respondent, providing a range of scores from 0–10, the ‘0’ score indicates no pain at all and the ‘10’ score indicates severe pain. Further, VAS score was graded as score 0 as No, mild as score as 1-3, moderate as score 4-6, and severe score as 7-10.23
Grading of therapeutic response:
-
Mild was rated as 1+, with a VSS score 0-15 and a VAS score of 0-3.
-
Moderate was rated as 2+, with a VSS score of 16-31 and a VAS score of 4-7.
-
Severe was rated as 3+, with a VSS score of 32-43 and a VAS score of 8-10.
2.6. Therapeutic outcome: Based on the improvement of the parameters before, during and after the trial, the response was graded as follows
Relieved: It was scored as 1 if the VSS score was 0-15 and the VAS score was 0-3.
Partially relieved: It was scored as 2 if the VSS score was 16-31 and the VAS score was 4-7
Not relieved: It was scored as 3 if the VSS score was 32-43 and the VAS score was 8-10.
Outcome parameters for evaluation of the efficacy of polyherbal formulation:
The outcome parameters included were the Vaginal Symptom Scale (VSS) or Vaginal Complaint Scale (VCS) to assess the severity of the sign and symptoms associated with vaginal discharge, Visual Analogue Scale (VAS) for pain intensity for low backache (LBA). The secondary outcome parameter was the safety parameters. The results were categorized into three groups i.e., relieved, partially relieved and not relieved. Assessment of safety was done by clinical assessment at every visit and biochemical assessment pre-and post-treatment.
Statistical Analysis:
Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented as mean±SD (Min-Max) and results on categorical measurements are presented in Number (%). The significance was assessed at a 5 % level of significance.
RESULTS:
Participant recruitment: Participant’s recruitment was carried out from March 2017 to November 2018. A total of 99 patients were initially screened, 17 patients refused to participate and 52 patients were excluded because of ineligibility and exclusion at the time of evaluation [(IUCD (n=13), OCPs (n=7), Lactating (n=4), pregnancy (n=2), hypertension (n=5), diabetes (n=8), endometriosis (n=3), fibroid (n=4), malignancy (n=2) and hypothyroid (n=4)]. Thus, 30 patients were enrolled.
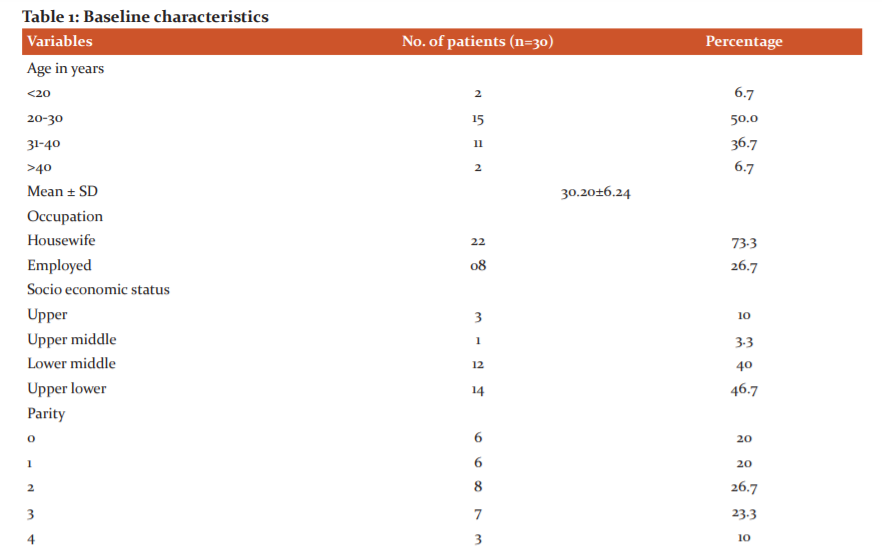
Baseline characteristics: The age of the patient was 30.20±6.24 years. In this study maximum respondents i.e., 14(46.7%) belonged to upper lower socioeconomic status as per Kuppuswamy’s socioeconomic scale, whereas 12(40%) to lower-middle, 3(10%) to upper and only 1(3.3%) to upper-middle class. The baseline characteristics are summarised in Table 1. The pap smear showed inflammatory smear in 18 (46.6%), bacterial vaginosis in 6 (20%) and candidiasis in 1 (3.3%) patients.
Primary outcome:
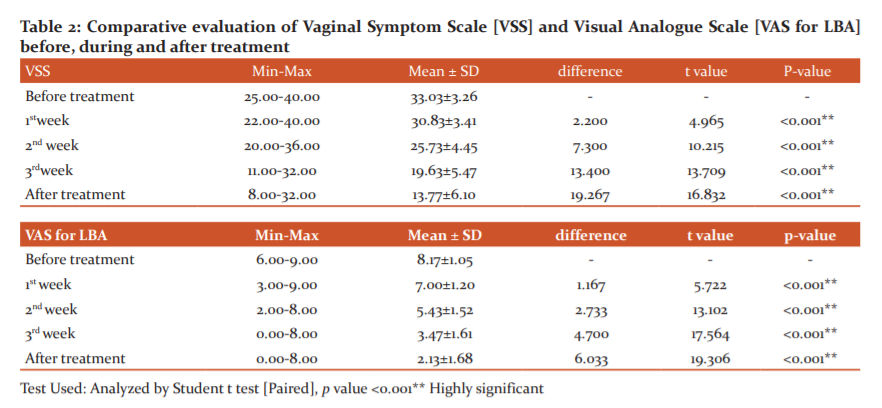
Vaginal Symptom Scale (VSS): The mean±SD of total VSS score before trial was 33.03±3.26. During the trial mean±SD of VSS score was 30.83±3.41, 25.73±4.45 and 19.63±5.47 in the first, second and third week respectively with significant difference (p <0.001**)(Table 2).
Visual Analogue Scale (VAS) for low backache: Pre-treatment, 4(13.3%) and 26(86.7%) patients had 4-6 and 7-10 VAS scores respectively. Post-treatment, 3(10%), 22(73.3%), 4(13.3%), and 1(3.3%) patient showed 0, 1-3 ,4-6 and 7-10 grades respectively. VAS score of 83.4% improvement was noted (p < 0.001**). Pre-treatment, the mean±SD of VAS was 8.17±1.05. During the trial mean±SD of VAS reduced to 7.00±1.20, 5.43±1.52 and 3.47±1.61 in the first, second and third week respectively and after trial mean± SD of VAS reduced to 2.13±1.68. Students ‘t’ test was used to analyse the effect and was found significant at p <0.001** (Table 2).
Secondary outcome:
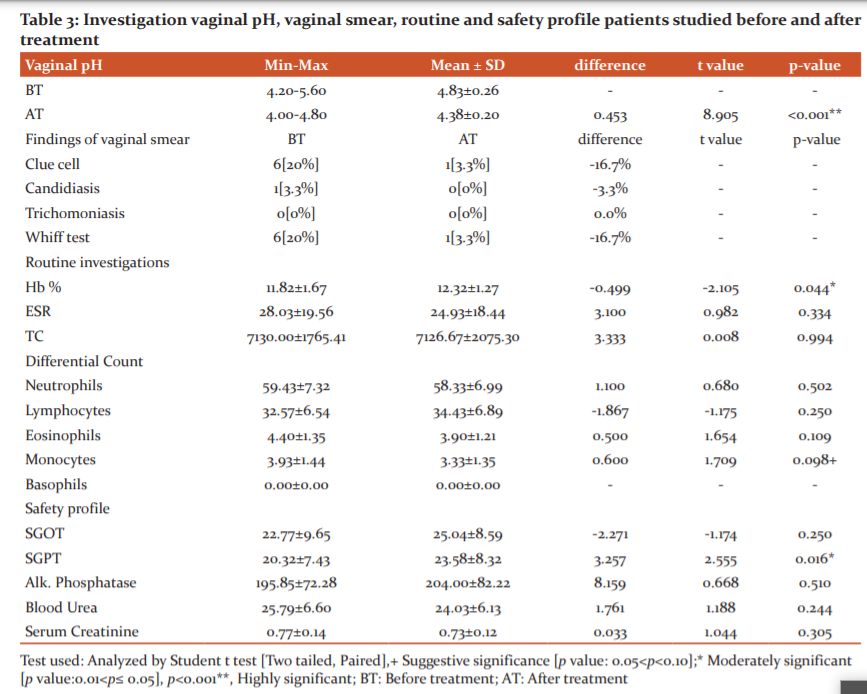
Safety profile: No significant difference was noted pre-and post-treatment in the routine and biochemical safety profile. (p>0.05) (Table. 3)
Therapeutic response: Assessment of the response was evaluated pre-and post-treatment. It revealed that after the treatment out of 30 patients, 25(83.33%) were relieved, 4(13.3%) partially relieved and only 1(3.3%) patient showed no response.
DISCUSSION:
The findings of this study showed that pre-and post-treatment, significant differences in VSS and VAS score was noted. Relief of symptoms was observed in 83.33% (n=25/30) patients. In Unani literature, it is described that accumulation of excessive flat (waste products) in the uterus leads to uterine infection that in turn causes a defect in nutritive faculty of the uterus resulting in leucorrhoea or vaginal discharge. Apart from the defective in nutritive faculty of the uterus, ghalbae akhlat Arba (predominance of humour) also causes vaginal discharge. P. mungo Linn and B. arundinacea Retz have cold-dry temperaments (barid wa yabis mizaj) and astringent property probably might have toned up the uterine nutritive faculty. Unani scholars surmised that O. latifolia L., P. mungo L., B. arundinacea Retz, P. oleracea L., P. lentiscus L. and B. Serrata Roxb have exhibited styptic, astringent, anti-inflammatory, anti-bacterial and anti-septic properties.13, 16 Further, these drugs contain flavonoids, phenolic acids, lipids, volatile oils, glucosides, and tannins.16
Further, the vaginal smear showed that 6 (20%) patients have bacterial vaginosis and 1 (3.33%) had candida infection before treatment and after treatment 5 (16.67%) showed absence of bacterial vaginosis and none of the patients had a fungal infection. This shows that the Unani formulation was effective in bacterial vaginosis, candida infection and reducing vaginal symptom scaleas B. Serrata showed anti-fungal activity20 and P. oleracea showed anti-microbial effect.21B. arundinacea Retz showed the anti-bacterial effect.24
Improvement in lower abdominal pain can be explained based on the analgesic effect of B. Serrata and P. oleracea L.20, 21 5- Boswellic acid has a COX-2 inhibitory activity.20 A study showed that B. Serrata significantly increased pain threshold force and time and pain tolerance force and time when compared to baseline and placebo with good safety and tolerability.19Anti-inflammatory property of B. Serrata, B. arundinacea Retz, and P. lentiscus L.17, 20, 21 possibly helped in resolving inflammation, thus relieved lower abdominal pain. Moreover, P. lentiscus L. possess wound healing activity and anti-trichomonas activity17 thereby these actions helped in reducing the inflammation. Further, these drugs were effective in reducing vaginal symptom scores and pain as they possess antioxidant properties. 20, 21
Safety profile:
Clinically no side effects were reported, and biochemical parameters showed no significant pre-and post-treatment, showing no side effects on blood, kidney and liver. Haematological, histopathological, and other observations revealed no toxicity of B. serrata,20 P. oleracea21 and B. arundinacea showed a protective effect.24
Future recommendation:
This study is limited by its small sample size, Nucleic Acid Amplification Tests [NAATs] and molecular methods like PCR for diagnosis of bacterial vaginosis and trichomoniasis, could not be employed due to paucity of time and cost. Hence, it might not reflect the real prevalence of this infection. Hence, further randomized controlled studies are recommended involving large sample size, and by using these precise investigations.
Conclusion:
Based on available data it can be concluded that the treatment with polyherbal formulation was effective in reducing vaginal discharge and its associated symptoms as it these drugs possesses antimicrobial, analgesic, anti-inflammatory, anti-trichomonas and antioxidant properties. The properties of these drugs are attributed to presence of phytoconstituents such as flavonoids, phenolic acids, glucosides, and tannins.
Conflict of interest statement: We declare that we have no conflict of interest.
Acknowledgement: The authors hereby acknowledge the Director and all the staff of the Department for their support in completing the trial. The authors also express thanks to all the participants of the study. Authors also acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Source of research funding: This study was supported by the contingency amount for postgraduate dissertation work, National Institute of Unani Medicine, Bengaluru, Ministry of AYUSH. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sector.
Competing interests: None to declare
Authors' contribution: Anjum AA has designed and planned the study, carried out the clinical trial, collated the data, interpreted the data and written and proof read the manuscript; Tabassum K has designed and planned the study, supervised the trial, interpreted the results. Sultana A was involved in analysing the data, drafting, critically reviewing and proof reading the manuscript.

References:
-
Kafle P, Bhattarai SS. Prevalence and factors associated with reproductive tract infections in Gongolia Village, Rupandehi District, Nepal. Advances in Public Health. 2016: ID 8063843: 1-5.
-
Rabiu KA, Adewunmi AA, Akinlusi FM, Akinola OI. Female reproductive tract infections: Understandings and care-seeking behaviour among women of reproductive age in Lagos, Nigeria. BMC Women's Health. 2010;10(8): 1-7.
-
Kumar P, Malhotra N. Jeffcoate’s Principles of Gynaecology, 8th ed. New Delhi: Jaypee Brothers Medical Publishers;2016; 23(4): 315-17.
-
Thulkar J, Kriplani A, Agarwal N, Vishnubhatla S. Aetiology & risk factors of recurrent vaginitis & its association with various contraceptive methods. Indian J Med Res. 2010; 131(1): 83-7.
-
Monif GR, Baker DA. Infectious diseases in Obstetrics and Gynaecology. London: The Parthenon Publishing Group; 2005;546-54.
-
Panda G, Mohapatra KB. Clinical effect of Kukkutanda Twak Bhasma in the management of Sweta Pradara. J Ayu. 2011;32(3):370-74.
-
Lakshmi V, Gupta RK. Ayurvedic Concept of Leucorrhoea: Sweta Pradara. Int J Ayur Pharma Res. 2014;2(3):119-23.
-
Polk J, Mattson S, Nyirjesy P. 28: Chronic cervicitis: presenting features and responses to therapy. Am J Obstet Gynaecol. 2015; 213(6):907.
-
Sánchez A, Rivera A, Castillo F, Ortiz S. Cervical erosion as result of infectious vaginitis. Eur J Exp Biol. 2012; 2(5): 1659-63.
-
Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bulletin of the World Health Organization. 2019;97(8):548-62.
-
Hemsel DL, Ledger WJ, Martens M, Monif GRG, Osborne NG, Thomason JL. Concerns regarding the centres for disease control’s published guideline for pelvic inflammatory disease. Clin Infect Dis. 2001;32(1):103-07.
-
Crossman SH. The challenge of pelvic inflammatory disease. J Am Fam Physician. 2006; 73(5): 859-64.
-
Khan A. Al-Akseer (Urdu trans.by Kabeeruddin). Vol II. New Delhi: Aijaz publishing house; 2003.p.715, 1382-84.
-
Kabiruddin H. Makhzanul Mufradat. New Delhi: Idarae Kitabus Shifa; 2007;153-54:168-69.
-
Tang D, Dong Y, Ren H, Li L, He C. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem Cent J. 2014; 8(4): 1-9.
-
Khare CP. Indian Medicinal Plants: An Illustrated Dictionary. Berlin: Heidelberg Springer; 2007;80(1):98-9.
-
Eldin HM, Badawy AF. In vitro anti-Trichomonas, vaginalis activity of Pistacia lentiscus mastic and Ocimum basilicum essential oil. J Parasit Dis.2015;39(3):465-73.
-
Kaikini AA, Dhande SR, Kadam VJ: Overview of Indian medicinal tree: Bambusa Bambos (Druce). Int Res J Pharm. 2013:4(8):52-6.
-
Prabhavathi K, Chandra US, Soanker, R, Usharani P. A randomized, double-blind, placebo-controlled, cross over study to evaluate the analgesic activity of Boswellia serrata in healthy volunteers using a mechanical pain model. Indian J Pharmacol.2014;46(5):475-9.
-
Sultana A, Rahman K, Padmaja AR, Rahman S. Boswellia serrata Roxb. a traditional herb with versatile pharmacological activity: a review. Int J Phar Res. 2013;4(6):2106-17.
-
Sultana A, Rahman K. Portulaca oleracea Linn. A global Panacea with ethno medicinal and pharmacological potential. Int J Pharm Pharm Sci. 2013;5(Suppl2): S33-S9.
-
Anderson M, Cohrssen A, Klink. K, Brahverd. Are speculum examination and wet mount necessary in patients with vaginal symptoms? J Am Fam Med. 2009; 22 (6):617- 24.
-
Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain). Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011; 63(11): S240-52.
-
Jaimik DR, Nimish LP, Ritesh GP, Jivani NP, Nayna MB. Phytopharmacological Properties of Bambusa arundinacea as a Potential Medicinal Tree: An Overview. J Appl Pharm Sci. 2011; 1(10): 27-31
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License