IJCRR - 13(11), June, 2021
Pages: 38-44
Date of Publication: 04-Jun-2021
Print Article
Download XML Download PDF
Solasodine with Coenzyme Q10 Supplementation Ameliorates High Fat Diet-Induced Metabolic Syndrome in Rats by Modulating Adipokines and Lipid Peroxidation
Author: Kumar R, Khan MI, Prasad M, Badruddeen, Akhtar J, Swarnalata N
Category: Healthcare
Abstract:Introduction: Metabolic Syndrome (MS) is a group of disorders including abdominal obesity, glucose intolerance or insulin resistance, dyslipidemia, hypertension, lipid peroxidation, and pro-inflammatory states. Solasodine and Coenzyme Q10 are acknowledged as potent antioxidants that prevented oxidative stress; a critical risk factor for the progression of MS. Objective: To evaluate the potency of solasodine with coenzyme Q10 on high fat diet-induced metabolic syndrome in rats by modulating adipokines and lipid peroxidation. Methods: Out of six groups (n=6), one group as a control (normal) received a standard diet for 16 weeks, and the other five groups were administered with a high-fat diet (HFD) only or with pioglitazone, coenzyme Q10, Solasodine and Solasodine with coenzyme Q10, (SDQ10) for the last 4 weeks. Results: Animals feeding with HFD for 16 weeks exhibited a significant increase in body weight, total cholesterol, triglyceride, and a decrease in HDL-cholesterol as well as developed glucose intolerance. It also raised lipid peroxidation and leptin level as well as lowered reduced glutathione and adiponectin level. Treatment with a combination of solasodine and coenzyme Q10 effectively reversed the above paradigm of metabolic syndrome induced by a high-fat diet. Besides, treatment with SDQ10 reduced the lipid peroxidation and enhanced the reduced glutathione as well as normalized the levels of leptin and adiponectin. Conclusion: The combined supplementation of solasodine and coenzyme Q10 reversed the effect of HFD intake on lipid peroxidation and adipocytokine levels and improve the symptoms of metabolic syndromeA
Keywords: Adiponectin, Dyslipidemia, Glucose Intolerance, Leptin, Oxidative stress
Full Text:
INTRODUCTION
Metabolic syndrome (MS) is a heaping of three or more disorders including abdominal obesity, glucose intolerance or insulin resistance, dyslipidemia, hypertension, lipid peroxidation, and pro-inflammatory states. Each of these understated disorders is a consequential risk factor that can lead to the development of heart disease and endothelial dysfunction. The increased high-calorie food intake and reduced energy expenditure due to a sedentary lifestyle contribute to the present rising popularity of obesity.1,2 Studies have exposed that HFD plays a critical role to intensify obesity, hyperglycemia, dyslipidemia, impaired insulin sensitivity or glucose intolerance, oxidative stress, and raised blood pressure, which is together clubbed as MS.3,4 Besides, adipose tissue-derived adipocytokines such as leptin and adiponectin are the important hormones that regulate body weight by regulating calorie intake and energy expenditure. Dysregulated secretion of these adipocytokines i.e. lower adiponectin and higher leptin may contribute to the progression of obesity and other metabolic disorders.5 Leptin inhibits appetite and decreased body mass by acting on the hypothalamus. However, a higher level of leptin was observed in most obese patients and animals but it fails to suppress feeding and decrease body mass, resulting in fat accumulation in adipose tissue as well as liver, muscle, and pancreas. This failure of leptin's effect on body tissue is called leptin resistance which may lead to the progression of obesity and insulin resistance.6
Solasodine (SD) is a principle aglycone (spiroketal) of solamargine and solasonine which are the most valuable steroidal glycoalkaloids, procured from different parts of solanaceous plants (family-Solanaceae).7 The earlier studies have exhibited that solasodine prevented lipid peroxidation (oxidative stress) and atherogenesis by reducing total cholesterol and LDL level in rats.8,9 Solasodine also contains several activities including anticancer, antiobesity, anti-inflammatory, antinociceptive, anticonvulsant, antifungal, immunomodulatory, and other activities on the central nervous system.10 In previous studies of acute toxicity, LD50 of SD was established as 2 g/kg in orally administered rats.11
Besides, Coenzyme Q10 (Q10) is acknowledged as an intracellular antioxidant that protects mitochondrial membrane proteins by removing free radicals and preventing lipid peroxidation.12 Q10 may decrease hyperinsulinemia, hyperglycemia, hyperlipidemia, and blood pressure which are the important components of MS.13 The acute toxicity study is also reported that the LD50 of Q10 was greater than 20 g/kg, supports the safety of Q10 for oral consumption.14
This study established that HFD intake for the 16 weeks leads to the onset of metabolic syndrome, through its effect on body weight, glucose tolerance, lipid profile, lipid peroxidation, and adipocytokines such as leptin and adiponectin level. This study also elucidates the potency of Solasodine with Coenzyme Q10 supplementation (SDQ10) against the HFD induced on lipid peroxidation and adipocytokine levels with improving the above paradigm of MS.
MATERIALS AND METHODS
Test Chemicals
A high-fat diet composed of (g/kg): Casein-250g, Cholesterol-10g, Lard-310g; dl-Methionine-3g, Yeast powder- 1g; Sodium chloride -1g; Pellet diet-365g was obtained from AIIMS, New Delhi. Solasodine and Coenzyme Q10 were obtained from Med Chem Express (LLC–USA) and Sigma Chemical (St. Louis. Mo.). All the chemicals used were of analytical grade.
Experimental animals
Wistar albino rats of either sex weighing 150-170 g were selected for the study. All animals were acclimatized at standard room temperature and an adequate light schedule of 12hrs light and 12 hrs dark with food and water ad libitum. The experimental protocol was approved by the institutional animal ethical committee of KNIMT, Faculty of Pharmacy, Sultanpur (KNIMT/PHAR/IAEC/18/04). All experiments were performed as per the guidelines of CPCSEA.
Experimental design
The experiment was performed on rats divided into 6 groups randomly, each consisting of 5-7 animals. Group I as control was provided with a standard (normal) diet for 16 weeks. The HFD was given to the remaining five groups for 16 weeks. Group II continued to supply with HFD while group III was treated with pioglitazone (10 mg/kg, orally), group IV with Q10 (50 mg/kg, orally), group V with SD (50 mg/kg, orally), and Group VI with a combination of SD and Q10 i.e. SDQ10 (25 mg/kg of each; total 50 mg/kg, orally) in the last 4 weeks of study. All drugs were administered as a suspension prepared by 1% Carboxymethylcellulose.
Measurement of Body, Organ Weights, and abdominal circumference
Each animal’s body weight was recorded before we begin our experiment & then weekly during the entire period of the experiment by weighing balance. When the study period was over, the animals were sacrificed & the weights of the liver, heart, and fat pad (adipose tissue) were measured. The abdominal circumference was measured every 4 weeks of the study periods.
Oral glucose tolerance test (OGTT)
After 16 weeks of HFD intake, OGTT was carried out in overnight-fasted animals by administration of glucose (2g/kg body weight) orally. Samples were taken before glucose administration and after glucose administration at 30, 60, 90,120 min respectively. The total area under the curve (AUC) in terms of mg/dl/h was calculated by the trapezoidal rule.15
Biochemical estimation
After 16 weeks of the study, Blood samples were taken from 12 hours of fasted animals via retro-orbital plexus and centrifuged at 2000 rpm for15 min to separate serum. Total cholesterol (TC) Triglycerides (TGs), and HDL-cholesterol in serum were determined by using Span diagnostic commercial kits. LDL and VLDL-cholesterol were also determined by using the formula of Friedelwald et al.16 The leptin and total adiponectin level were determined with rat leptin ELISA kit (BioVendor, Brno, Czech Republic) and adiponectin ELISA kit (Alpco diagnostics Salem, USA) respectively.
The isolated liver was homogenized with phosphate buffer (pH7.4), and the homogenate was used for the estimation of lipid peroxidation (TBARS) and reduced glutathione (GSH) as antioxidant activity.
Histopathology
Isolated liver and heart tissue samples were kept in 10 % formalin neutral buffered solution, Section (5μm) were cut using rotary microtone and stained with hematoxylin & eosin. The sections of all tissues were studied under an optical microscope to determine the level of tissue damage.
Statistical analysis
All data were presented as mean ± S.E.M. To evaluate the differences among groups, one-way ANOVA followed by post hoc (Tukey's) multiple comparison tests was used via Graph Pad Prism-8 computer program. The probability p<0.05 value was considered as standard for statistical significance.
RESULT
Effect of SDQ10 on Body Weight, organ weight, and abdominal circumference
As in Table 1, All HFD-fed animals showed a significant raise (***p<0.001) in body weight, body weight gain, and abdominal circumference as compared to normal diet-fed animals. During the treatment period of SD and SDQ10 the body weight and body weight gain, as well as abdominal circumference was found to be significantly (###p<0.001) reduced in HFD fed animals. A significant increase in weight (absolute) of the liver, heart, & Adipose tissue was found in the HFD group as compared to the normal group. Treatment with combination SDQ10 and pioglitazone significantly decreased the weight of liver & adipose tissue compared to the high-fat diet administered group. However, no significant impact on the weight of the liver, heart, and adipose tissue was found in the group treated with solasodine or coenzyme Q10 alone.
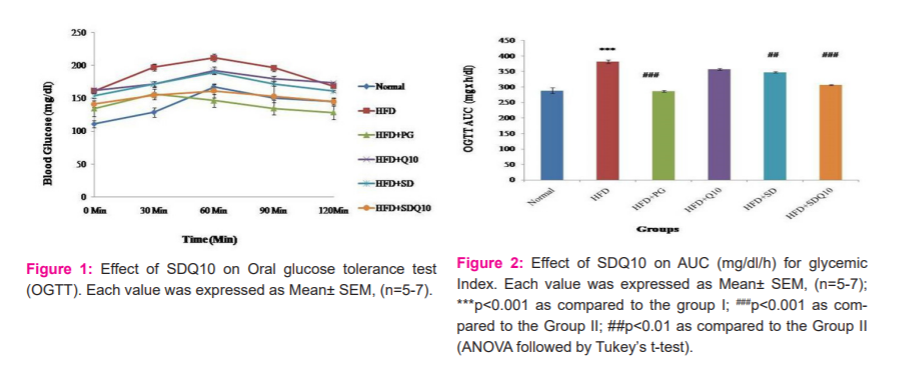
Effect of SDQ10 on OGTT& AUC (mg/dl/h) for blood glucose
Blood glucose was influenced by the time during the performance of OGTTs. As compared to the normal group, a significant increase in AUC (glycaemic response) was exhibited in the HFD group. However, Solasodine and its combination with Q10 as well as standard pioglitazone presented a significant decrease in AUC compared to rats fed with HFD (Figures 1 and 2).
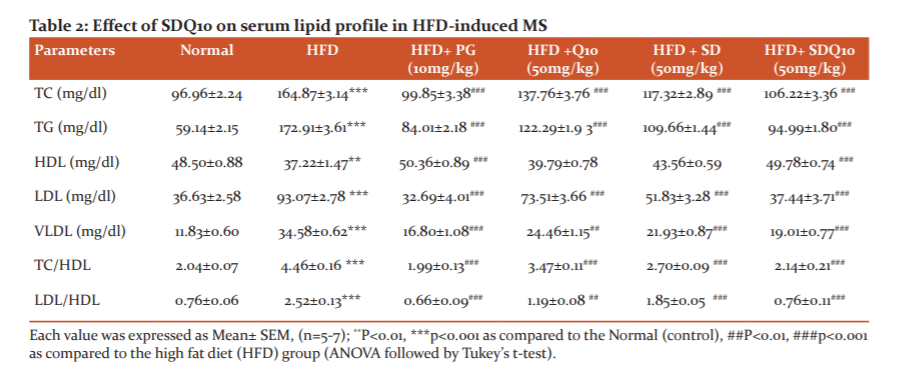
Effect of SDQ10 on lipid profile
Lipid profiles in serum are exhibited in Table 2. As compared to the normal group, atherogenic index as well as concentrations of TC, TG, LDL-C, and VLDL-C in serum were significantly (p<0.001) increased in the HFD group. As compared to the HFD group, the concentration of these parameters was significantly reduced in SD and SDQ10 treated animals. Although the administration of solasodine alone did not increase the HDL level, treatment with SDQ10 combination significantly (p<0.001) increased the level of HDL.
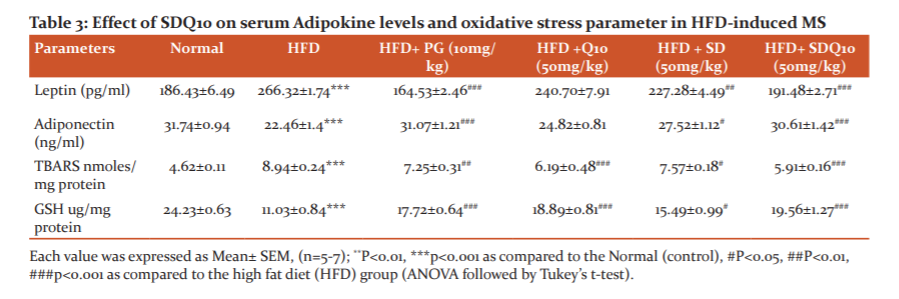
Effect of SDQ10 on serum Adipocytokine levels and Oxidative stress parameter
Feeding of high-fat diet significantly raises the serum leptin concentration as compared to the untreated control group. However, Increase in serum leptin level was prevented in SD and SDQ10 treated groups. Also, the adiponectin level of the HFD group was found to be reduced significantly as compared to the normal group. Comparing with HFD fed animals, SD (p<0.05) and combination SDQ10 (p<0.001) treated animals showed a significant increase in adiponectin level. In addition, HFD administration for 16 weeks raised the TBARS level and significantly reduced the level of antioxidant enzyme i.e. reduced glutathione (GSH). However, Treatment with SD (p<0.05) and SDQ10 (p<0.001) significantly enhanced the level of GSH as well as reduced the TBARS (lipid peroxidation) level (Table 3).
Effect on Histopathological examination
In histopathology of the liver, macrovascular fatty changes in hepatocytes were observed in the HFD group. Treatment with SDQ10 and standard pioglitazone leads to the protection of macrovascular fatty change in hepatocytes (Figure 3). In histology of the heart, infiltrating inflammatory cells with cardiomyocyte disorganization and interstitial fibrosis were observed in the HFD group, whereas no marked pathological changes occur in the SDQ10 and pioglitazone treated group (Figure 4).
DISCUSSION
Metabolic syndrome (MS) is a cluster of risk factors including obesity, dyslipidemia, glucose intolerance or insulin resistance, and hypertension. The present study provided exciting insights on the development of obesity and the potential of solasodine with Coenzyme Q10 supplementation (SDQ10) in regulating the lipid peroxidation and adipokines level with the condition of obesity in the high fat diet-fed rats. In our study, the pattern of food intake in the different groups reflected on the body weight, organ weight (liver, heart, & adipose tissue), and abdominal circumference of the animals. As compared to the control group, a significant increase in body weight, organ weight, and abdominal circumference of the HFD group was recorded. In similar studies conducted earlier, it has been reported that HFD fed rats gained more body weight, organ weight, and abdominal circumference as compared to control rats.17-19 Treatment with SD and SDQ10 suppressed the increased body weight and abdomen circumference induced by HFD. However absolute liver weight and weight of adipose tissue were significantly reduced in SDQ10 and standard pioglitazone treated group.
In the study of the oral glucose tolerance test, HFD feeding induced a significant increase in AUC for glucose as compared to normal feeding rats. SD and SDQ10 effectively lowered the raised AUC and brought it close to a normal value within four weeks of treatment, proposing the treatment of glucose intolerance. It has been shown earlier that, the AUC of glycemic response was significantly greater in HFD feeding rats.20
The increased serum cholesterol, TGs, LDL, VLDL as well as reduced HDL in HFD fed rats have been reported in the development of dyslipidemia, one of the important risk factors to inducing obesity.21 We found in our studies that SD and SDQ10 treated groups had significantly reduced the levels of TC, TG, LDL-C, VLDL, and atherogenic index. However, only SDQ10 and standard drug pioglitazone administration had significantly increased the HDL level. This result suggests that solasodine supplemented with Q10 showed better lipid profiles compared to solasodine alone. However, the previous study reported the lipid-lowering activity of solasodine in HFD fed rabbits.9
Leptin and adiponectin hormones act upon the brain to regulate energy balance by regulating calorie intake and energy expenditure.22,23 In the present study, leptin resistance was evidenced by a significant rise of the serum leptin in the HFD group, compared to the normal group. Meanwhile, Treatment with solasodine and its combination SDQ10 significantly ameliorated leptin resistance. In this study also, a significant reduction in the adiponectin level was recorded in HFD group, which was increased by treatment with SD and SDQ10 in the high fat diet-fed rats. Previous studies reported that a reduced adiponectin level was observed in obese animal models or HFD fed animals, while higher adiponectin was reported in animals with low body weight. These studies indicated that food composition and lifestyle influence body weight by controlling leptin and adiponectin levels.24,25 Hence, lower levels of leptin, a higher level of adiponectin, as well as reduced body weight and weight of fat pad, which was observed in treated groups, may explain the basis of its reversal of metabolic syndrome.
In this study, HFD produces oxidative stress as evidenced by a significant increase in TBARS level and reduced level of reduced glutathione (GSH). Chronic consumption of a high-fat diet leads to excessive production of reactive oxygen species (ROS), which induces oxidative stress. Many studies have reported earlier that oxidative stress is produced from the inadequate antioxidant status and increased peroxidation markers (TBARS level) in the blood and liver of rats fed HFD. Oxidative stress and its mediators have reportedly implicated in the induction of MS.26,27In this study, SD and SDQ10 treated groups produced a significant increase in GSH level and reduced the TBARS level. This result justified the antioxidant property of solasodine and coenzyme Q10, which reported in previous studies also. Hence, the antioxidant property of SDQ10 can be used in the treatment of oxidative stress-induced obesity.
Fatty liver and heart dysfunction may also be responsible for the development of obesity and cardiovascular disorder.28,29 Impaired metabolism or excess formation of fat in the body may lead to the accumulation of fat in the liver that can develop fatty liver or hepatic steatosis. In the present histological study, we observed that the SDQ10 combination had effectively minimized the content of fatty droplets in hepatic tissue as well as inflammation and fibrosis in heart tissue in animals fed with HFD. Hence the SDQ10 combination has a potential effect in ameliorating the fatty liver and inflammation in HFD fed rats.
CONCLUSION
From our results, it can be concluded that Solasodine with Coenzyme Q10 supplementation (SDQ10) improved the related markers of metabolic syndrome, including obesity, lipid profile, glucose intolerance, increased oxidative stress, and circulating adipokine levels i.e. leptin and adiponectin. Hence, combined supplementation of solasodine and coenzyme Q10 reversed the effect of high-fat diet intake on lipid peroxidation and adipokine levels with improving the symptoms of metabolic syndrome.
ACKNOWLEDGEMENT
The authors are thankful to Integral University Lucknow, & KNIMT-Faculty of Pharmacy, Sultanpur for providing facilities regarding research work, suggestions for improvement in manuscript, and special thanks to the research community of Integral University for providing manuscript number IU/R&D/2020-MCN-0001006.
FUNDING: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST: No conflict of interest
AUTHOR’S CONTRIBUTION:
Kumar R and Khan MI have participated in the conception and design, or approval of the final version. Prasad M and Badruddeen have participated in the analysis and interpretation of data.
Juber A and Swarnalata N. have contributed to drafting the article or revising it critically for important intellectual content.


References:
-
Gregory JW. Prevention of Obesity and Metabolic Syndrome in Children. Front Endocrinol. 2019; 10(669):1-9.
-
Payab M, Ranjbar SH, Shahbal N, Qorbani M, Aletaha A, Aminjan HH et al. Effect of herbal medicines in obesity and metabolic syndrome: Asystemic review and meta-analysis of clinical trials. Phytother Res. 2019;17:1-20.
-
Xu RY, Wan YP, Tang QY, Wu J, Cai W. The effects of high fat on central appetite genes in Wistar rats: a microarray analysis. Clin Chim Acta 2008;397:96–100.
-
Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modelling the metabolic disorders of human obesity in rodents. Obesity 2007; 15:798–808.
-
Wu Q-M, Ni H-X, Lu X. Changes of adipokine expression after diabetic rats received sitagliptin and the molecular mechanism. Asian Pac J Trop Med 2016;9(9):893–897.
-
Handjieva-Darlenska T. and Boyadjieva N. The effect of high-fat diet on plasma ghrelin and leptin levels in rats. J Physiol Biochem 2009;65(2):157–164.
-
Hussain T, Gupta RK, Sweety K, Khan MS, Hussain MS, Arif Md, et al. Evaluation of the antihepatotoxic potential of Solanum xanthocarpum fruit against antitubercular drugs induced hepatopathy in experimental rodents. Asian Pac J Trop Biomed 2012: 454-60.
-
Sharma T, Airao V, Panara N, Vaishnav D, Ranpariya V, Sheth N, et al. Solasodine protects rat brain against ischemia/reperfusion injury through its antioxidant activity. Eur J Pharmacol. 2014;725:40-46.
-
Dixit VP, Varma M, Mathur NT, Mathur R, Sharma S. Hypocholesterolaemic and antiatherosclerotic effects of Solasodine. (C27H42O2N) in cholesterol-fed rabbits. J Phytother Res 1992; 6(5): 270-273.
-
Patel K, Singh RB, Patel DK. Medicinal significance, pharmacological activities, and analytical aspects of Solasodine: A concise report of current scientific literature. J Acute Dis 2013;23:92-98.
-
Chauhan K, Sheth N, Ranpariya V, Parmar S. Anticonvulsant activity of Solasodine isolated from Solanum sisymbriifolium fruits in rodents. Pharm Biol 2011;49:194-199.
-
Kettawan AT, Takahashi R. Kongkachuichai, Kishi T, Okamoto T. Protective Effects of coenzyme Q10 on Decreased Oxidative Stress Resistance Induced by Simvastatin. J Clin Biochem Nutr 2007; 40: 194–202.
-
Singh RB, Rastogi, Rastogi V, NiazMA, MadhuSV, Chen M.et al. Blood pressure trends, plasma insulin levels and risk factors in rural-urban elderly populations of north India. Coronary Art Dis 1997; 8: 463–468.
-
Fu X, Ji R, Dam J. Acute, subacute toxicity and genotoxic effect of CoQ10 in mice and rats. Regul Toxicol Pharmacol 2009; 53:1–5.
-
Liu J, Zhang HJ, Ji BP, Cai SB, Wang RJ, Zhou F. A diet formula of Puerariae radix, Lycium barbarum, Crataegus pinnatifida, and Polygonati rhizome Alleviates insulin resistance and hepatic steatosis in CD-1 mice and HepG2 cells. Food Funct 2014;5(5):1038-49.
-
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502.
-
Amin KA, Nagy MA. Effect of Carnitine and herbal mixture extract on obesity induced by high-fat diet in rats. Diabetol Metab Synd 2009;12:1:17.t
-
Nammi S, Sreemantula S, and Roufogalis BD. Protective effects of ethanolic extract of Zingiber officinale Rhizome on the Development of Metabolic Syndrome in High-Fat Diet-Fed Rats. Basic Clin Pharmacol Toxicol 2009;104:366–373.
-
Kim CM, Yi SJ, Cho IJ, and Ku SK. Red-Koji Fermented Red Ginseng Ameliorates High Fat Diet-Induced Metabolic Disorders in Mice. Nutrients 2013;5:4316-4332.
-
Honors MA, Hargrave1 SL, and Kinzig KP. Glucose Tolerance in Response to a High-Fat Diet Is Improved by a High-Protein Diet. Obesity 2012; 20(9):1859-65.
-
Kumar P, Bhandari U, and Jamadagni S. Fenugreek Seed Extract Inhibit Fat Accumulation and Ameliorates Dyslipidemia in High Fat Diet-Induced Obese Rats. Bio Med Research Int 2014;5:1-11.
-
Zhou CJ, Huang S, Liu JQ, Qiu SQ, Xie FY, Song HP, et al. Sweet tea leaves extract improves leptin resistance in diet-induced obese rats. J Ethnopharmacol 2013;45:386–392.
-
Barnea M, Shamay A, Stark AH, Madar Z. A high-fat diet has a tissue-specific effect on adiponectin and related enzyme expression. Obesity 2006;14(12): 2145–2153.
-
Hotta K, Funahashi T, Arita Y, Takahashi M, Matuda M. Plasma concentration of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. J Clic Endocrinol Metab 2001;86:1930–1935.
-
Milan G, Granzotto M, Scarda A, Calcagno A, Pagano C. Resistin and adiponectin expression in visceral fat of obese rats effect of weight loss. Obes Res 2002;10(11):1095–1103.
-
Milagro FI, Campion J, Martinez JA. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity 2006;14:1118−1123.
-
Malam PP, Amin AJ, Zala AC, Navadiya VM, Patel D, Patel DA. Study of oxidative stress parameters in type-II diabetes mellitus and their correlation with blood glucose level. Int J Cur Res Rev 2016;8(13):31-34.
-
Ganesan K, Sukalingam K, and Xu B. Solanum trilobatum L. ameliorate Thioacetamide-Induced Oxidative Stress and Hepatic Damage in Albino Rats. Antioxidants 2017;6(68):1-10.
-
Panchal SK, Poudyal H, Iyer A, Nazer R, Alam MA, Diwan V, et.al. High-carbohydrate, High-fat Diet-induced Metabolic Syndrome and Cardiovascular Remodeling in Rats. J Cardiovasc Pharmacol 2011;57(5):611–624.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License