IJCRR - 13(9), May, 2021
Pages: 162-167
Date of Publication: 07-May-2021
Print Article
Download XML Download PDF
A Clinicopathological Study and Diagnosis of Helicobacter Pylori by Special Stains in Gastric Biopsies\?Is it Noteworthy?
Author: Sonti Sulochana, Vithya Priya K, Muhamed Yusup A
Category: Healthcare
Abstract:Background: Helicobacter pylori are a gram-negative, curved bacillus, found in the gastric mucosa and play an important role in the development of gastritis, gastric ulcer, and gastric malignancy. More than half the world's population is infected with this organism. Because of this significant pathogenic diagnosis, H. Pylori is very essential. Various invasive and non-invasive methods are available for the identification of H.pylori. Histopathological identification of H.pylori can be improved with the use of special stains Objective: This study aimed to evaluate the efficacy of special stains like acridine orange, cresyl violet, Giemsa, toluidine blue, and warthinstarry stain in the identification of H.pylori compared to the routine H &E stain (Haematoxylin and eosin). Methods: A Case Series Analysis of fifty gastric biopsies (twenty-five cases are positive for helicobacter pylori by Haematoxylin and Eosin stain and twenty-five gastric biopsies are negative for H pylori by the same method taken as controls) were included from March 2019-August 2019. Histological sections of gastric biopsy slides were stained with the H&E, and five special stains and Sensitivity, Specificity was calculated Results: Out of 50 cases studied, the sensitivity of five special stains was excellent in both positive and negative cases of H.pylori diagnosed by H&E stain. Conclusion: The most reliable stains are Cresyl violet, Acridine orange, Modified Giemsa, and Toluidine blue in terms of posi�tivity, cost-effectiveness, time-consumption and can be used as an adjunct to standard H&E stain for definitive confirmation of H.pylori in gastric biopsies.
Keywords: Activity, Gastric biopsies, H. pylori, Inflammation, Stains
Full Text:
INTRODUCTION
Helicobacter pylori are gram-negative, microaerophilic, spiral organism which inhabits the gastric mucosa. H.pylori-related diseases are the most prevalent in the world especially in the subcontinent of India.1Itis an important etiological factor of numerous benign, premalignant and malignant lesions like peptic ulcer, chronic gastritis, intestinal metaplasia, gastric carcinoma, and Mucosa-associated lymphoid tissue lymphoma[MALT].2 It was first discovered by Warren and Marshall in 1983. The occurrence of H.pylori infection is more common in developing countries when compared with developed countries and the incidence increases with age.3 But most of the cases are seen in adults mainly due to lifestyle changes and associated co-morbid conditions.
The diagnosis of H. pylori in gastric mucosal biopsies is important because of its pathogenicity. For an accurate diagnosis of H.pylori, multiple biopsies are required from different sites such as two corpora and two antral specimens.4,5 Nowadays various methods are available to detect H. pylori in gastric specimens.6 To date, several studies have found that histopathological examination remains the best technique for H. pylori identification.7The culture method is highly specific for H. pylori detection, but it is a strenuous and time-consuming method. PCR is extremely sensitive and very specific when compared with any other prevailing diagnostic methods. It also plays a role in detecting mutations seen with antimicrobial resistance, typing of organisms, and testing of virulence of organisms.8
Antibody test detects the presence of IgG antibodies specific to H.pylori in whole blood, serum, and urine, usually seen around twenty-one days of infection which persist for a long time even after eradication.9 Urea Breath test identifies the organism through H.pylori urease activity. After consuming urea labeled either with radioactive isotope 14C or nonradioactive isotope 13C, in the presence of organisms, there will be the production of labeled carbon dioxide which is measured in expired breath.10 The sensitivity and specificity of H&E slides increased with all levels of observers.11 When the magnifying field is large and the bacterial count is high, routine H&E staining is adequate to establish the presence of the organism. But if the density of the micro-organisms is low and when atrophic mucosal changes are present, special stains are required. IHC staining is also highly sensitive and reliable and advantageous in patients partially treated for H. pylori gastritis, which can result in atypical (including coccoid)forms, which may mimic bacteria or cell debris on H&E preparations The major advantages of IHC stain is less screening time and high specificity. The morphological changes in gastritis are activity, chronic inflammation, atrophy, intestinal metaplasia, and H. Pylori density are graded as mild (G1), Moderate (G2), and severe (G3) by the Updated Sydney System.
Activity is defined as the additional presence of neutrophils. It is an indicator of acute inflammation and H.pylori infection. In H.pylori-positive cases, "pit abscesses" are formed by neutrophilic infiltration in the epithelium, lamina propria, and in the foveolar lumen. The severity of the infection and the level of mucosal damage usually correspond to the density of neutrophils in the epithelium. The presence of neutrophils in post-treatment biopsies is highly suspicious of H. pylori infection. So in this condition, the use of immunostains or special stains for detecting H. pylori is important.
Chronic gastritis is marked by the uniform infiltration of superficial and/or deep lamina propria by lymphocytes, monocytes, eosinophils, plasma cells, and mast cells. It is graded as mild, moderate, and severe. Even after complete eradication of H. pylori, chronic inflammatory cells take several years to disappear or become normal in gastric mucosa. Atrophy refers to the loss of gastric mucosal glands which leads to mucosal thinning or the presence of intestinal metaplasia in the antral epithelium. Metaplastic change in gastric antral and/or fundic mucosa is caused by intestinal goblet cells, absorptive cells, and Paneth cells.
This study aimed to compare the efficacy of each special stain. Only a limited number of researches have examined the sensitivity and specificity of various staining techniques.12No studies have compared the special stains in h.pylori negative cases, to our knowledge only we have done this study.
MATERIALS AND METHODS
A Case Series Analysis of 50 gastric biopsies (positive and negative cases of H.pylori by H& E stain) was studied in the Department of pathology, saveetha medical college and hospital, March 2019-August 2019. Endoscopic biopsies were taken from the antrum, body, and other sites were included in the study, and gastrectomy specimens were excluded. The clinical details of the cases were accessed from biopsy requisition forms. The tissues were fixed in 10% formalin, processed and the sections were stained with hematoxylin and eosin(H&E) and five special stains.
Methods of Special stains
Modified Giemsa staining
To prepare the stock solution of Giemsa, 4 grams of stain powder was dissolved in 250 ml glycerol at 60°C with regular shaking. Add 250 ml of methanol was added, shook the mixture, and allow to stand for 7 days. The working Giemsa stain was prepared by adding 4ml of Giemsa stock solution to 96 ml of Acetate buffered distilled water(pH6.8). Sections are rinsed in buffered distilled water - a pH of 6.8. The working Giemsa stain is added to the specimen and left undisturbed for the whole night. Then rinsed in distilled water. Then rinsed in 0.5% aqueous acetic acid till the section becomes pink. Dehydrated with alcohol, cleared and mounted.
Toluidine blue staining
The toluidine blue solution contains toluidine blue in pH 6.8 phosphate buffer, add Sorenson's phosphate buffer, pH 6.8 - 50 ml, and 1% aqueous toluidine blue -1 ml. Stained with buffered toluidine blue for 20 minutes, washed with distilled water. Dehydrated and mounted.
Warthin-starry silver staining
The Warthin-starry silver staining solution contains acetate buffer, pH 3.6, sodium acetate 4.1 g, acetic acid 6.25 ml, distilled water 500 ml,1% silver nitrate in pH 3.6 acetate pH. Sections are immersed in a slightly acidified aqueous solution of silver nitrate and kept for 30 to 60 minutes. Immersed in a freshly made handmade reducing solution containing hydroquinone, gelatine, and a low concentration of silver nitrate. Rinsed in tap water for several minutes at 55–60°C, then in buffer at room temperature. Dehydrate, clear, and mount.
Acridine orange staining
The reagents used in acridine orange staining are acridine orange (CAS 10127-02-3)-0.1 g, 0.2 M,acetate buffer (pH 4.0) -1000.0 ml. The slide is fixed with absolute methanol for two minutes or with heat. Acridine Orange stain is flooded in the slide and kept undisturbed for two minutes. Rinsed with water and allowed to dry. Examined under 40X magnification using a fluorescent microscope.
Cresyl violet staining
Cresyl violet staining solution contains cresyl violet (acetate) -0.1 g, distilled water -75 ml and the working solution contains cresyl violet solution- 6 ml ,acetate buffer solution pH 3.6- 50 ml.Filter with 0.1% cresyl violet acetate onto a slide or into a Coplin jar for 5 minutes. Rinsed in distilled water. Blotted, dehydrated rapidly in alcohol, cleared, and mounted.
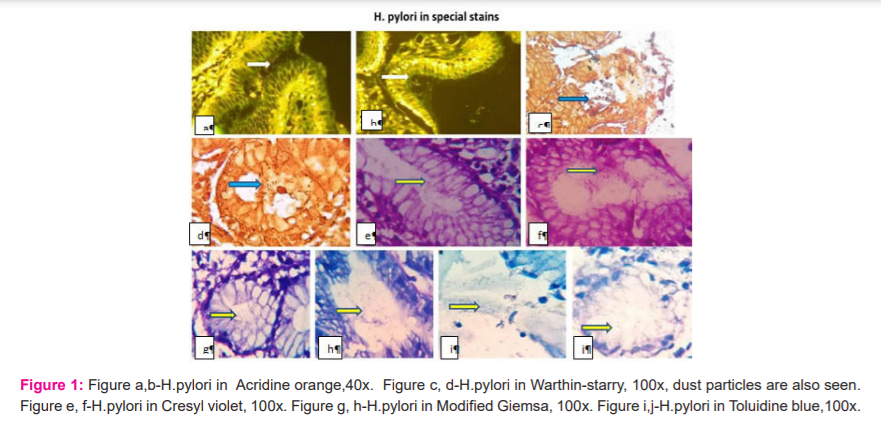
All cases were evaluated microscopically under the oil immersion objective(1000X, except acridine orange) for the presence of H.pylori. The H.pylori appeared as pink in H&E stain, Dark blue against a pink- pale blue background in modified Giemsa stain, dark blue against a variably blue background in toluidine blue stain, black against the golden yellow background in Warthin-starry silver stain, bright orange against a green-fluorescing or dark background in Acridine Orange stain and finally blue-violet in shades of blue-violet background in Cresyl violet staining (Figure 1)
All the data obtained were entered into a master sheet in Microsoft Excel. The findings were compared and sensitivity, specificity was calculated. The study was approved by the institutional ethics committee, SMC/IEC/2019/07/003
RESULTS
Out of 50 cases of gastric biopsies, 25 cases were H. pylori positive and 25 cases of H.pylori negative case controls in H&E staining were included in the study. The males (56%)are affected more than females (44), 2:1 ratio, the incidence is high in the age group ranging from 21 to 60 years.
Clinical diagnosis/symptoms
The most common clinical presentation is gastritis, dyspepsia, and acid peptic disease. The rare clinical presentation is gastric outlet obstruction, GERD, gastric ulcer, and gastric carcinoma (Table 1). To date, Histopathology and culture were the gold standards for the diagnosis of H.pylori, but the culture was not utilized nowadays because it takes a long time and also the availability of more advanced invasive and non-invasive procedures. H.pylori can be detected routinely by H&E stain, but when the density was low; its identification can be highly supplemented by special stains. In all positive and negative cases, five special stains were done. All the special stains showed a statistically equivocal result in H.pylori positive cases and less than 50% positivity in H.pylori negative cases in H&E staining (Table 2).
Microscopic findings
Activity, inflammation, atrophy, and H.pylori density were categorized by the updated Sydney system. In this study, out of 50 cases, mild activity in 22 (44%)cases, moderate activity in 26 cases (52%), and severe activity in 2 cases (4%). The intestinal metaplasia was seen in 7 cases (14%), chronic inflammation(lymph plasma cells and lymphoid follicles) in 12 cases (24%), and mixed inflammation (neutrophils, lymph plasma cells, and eosinophils) in 38 cases(76%). Depending upon the activity, the positivity of H.pylori in mild activity was 21, 18, 15, 12, and 5 cases and in moderate activity18, 21, 17, 20 and 10 cases were in acridine orange, cresyl violet, modified Giemsa, toluidine blue, and warthin starry stain respectively. In the H&E stain, 8 and 14 cases were positive for mild and moderate activity. In all stains positivity was increased in moderate activity (Figure 2) So the sensitivity was more in acridine orange followed by cresyl violet, Giemsa, toluidine blue, and low sensitivity, and more specificity was seen in warthin-starry stain (Tables 2,3). In our study, we compared all special stains with H&E stain(in positive, negative cases) depending upon the staining quality for interpretation of H.pylori, cost, and staining time. The most reliable stains were acridine orange, cresyl violet, Giemsa stain, and toluidine blue. The most time-consuming, complex, and expensive stain was the Warthin-starry stain (Table 4).
DISCUSSION
Helicobacter pylorus is a gram-negative, spiral organism that colonizes the gastric mucosa. H.pylori revives in the acidic medium of the stomach and burrows into the mucus layer, because of its helical shape. The common site of biopsy for the detection of H. pylori is the antrum because the colonization of bacilli is more severe than in the body. In our study, 47(94%) cases were obtained from the antrum and 3 (6%) cases were from lesser curvature.
The most common age of occurrence is between 21 and 41 years. Comparison of the male and female distribution of helicobacter pylori infection with other studies showed (Table 5). All studies showed males were commonly affected, except Adisa et al., and studies showed females were more affected than males.
Histopathology and culture were the gold standards for the diagnosis of h.pylori, but the culture was not doing nowadays because it takes more time to get results and also the availability of various invasive and non-invasive procedures. This study aimed to compare the efficacy, cost-effectiveness, and time of each special stain in both positive and negative cases diagnosed by H&E staining. The number of bacteria in the specimen determines the sensitivity of the test. The outcome of every test result depends on the hands of experienced has good sensitivity and specificity.13 In the present study, the sensitivity of acridine orange showed 96% accuracy which is the same as Haqqani MT, Langdale-Brown, et al and Gowsik K.13,14 They conducted two different studies in 1998 and have mentioned that Acridine Orange is not specific but 100% accurate and a study by Rotimi et al in 2000 also suggested the same.15 It is an easy and simple procedure to perform, but most of the labs do not have a fluorescence microscope. Kaur et al. in their study observed Toluidine Blue stain was cheap and easily applicable and consumed in only 4 minutes.16 The sensitivity and specificity were less when compared with MG, but in the present study, it was almost equivocal where sensitivity was 80% and specificity was 64% in TB and 84% and 60% in MG.
Previous studies et al found that H&E stain was cost-effective to use as they are routinely performed for the evaluation of gastric biopsies.16In our laboratory, we are also routinely used hematoxylin stain for H. Pylori detection in the majority of cases. However, in a small number of cases, an immune-histochemical stain can be particularly useful in severe active gastritis in which no H.pyloricould be detected on hematoxylin stains, to avoid the false-negative results, and for the follow-up, biopsies to confirm the absence of H. pylori.17 Wilkins in her study, said that increase in staining time of hematoxylin can give good results, but the sensitivity is low due to the lack of contrast between the bacteria and surrounding tissues, and specificity is also low due to the non-specific staining of the non-HP bacteria.16
A study done by Fiaz Ahmad al alshowed, 68% were positive in Giemsa and 76% were positive in Cresyl fast violet. Cresyl fast violet is a good stain for the diagnosis of H. pylori gastritis.18 In our study, the sensitivity of cresyl violet was 92% and 84% in Giemsa. Sulakshana et al. showed that the sensitivity of Warthin - Starry stain was the same as that of Giemsa, but Ashton et al have shown that the sensitivity is higher than that of Giemsa and the disadvantage of this stain is complex, not reproducible, and difficult to interpret because of nonspecific staining of mucus, and water bath contaminants.19,20 In our study, the sensitivity was low(52%) and high specificity (84%) when compared with all special stains. It was true, the same problem happened in our study. It was very expensive, complex, and suitable when the bacterial load is more and less reliable because of more granular and fibrillary artifacts.
However, hematoxylin and Eosin stains can be used as a standard procedure for initial screening. Even though IHC is a gold standard, the special stains are more useful in the diagnosis of H.pylori when it was not detected by H&E. These special stains were especially useful in small setup laboratories due to lack of facilities for Immunohistochemistry (IHC) and easy to perform, less expensive.
CONCLUSION
The routinely used H& E stain is cost-effective, easy to use. Positive and negative cases ofH.pylori were detected by Haematoxylin and Eosin, and compared with Giemsa, Cresyl violet, Toluidine blue, Acridine orange, and Warthin-Starry special stains. The most reliable stains are Cresyl violet, Acridine orange, Modified Giemsa, and Toluidine blue in terms of positivity, cost-effectiveness, time-consumption and can be used for definitive identification of Helicobacter Pylori. The sensitivity of five special stains was good(>90%) in all positive cases and > 50% positivity in negative cases, except Warthin- Starry stain has high specificity and low sensitivity in our study. So, we conclude that H.pylori can be identified easily with a careful examination by using any stain. In our study, the highly sensitive stain was cresyl violet. So we propose, Cresyl violet can be used for routine histological diagnosis of Helicobacter pylori in adjunct with H&E. Use of Acridine orange was limited, it required immunofluorescence microscope attachment,
Author Contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Conflict of interest: None.
Acknowledgement: We all thank our technicians who helped in this project.
References:
1. Ramakrishna BS. Helicobacter pylori infection in India: the case against eradication. Indian J Gastroenterol 2006; 25(1):25-8.
2. Garg B, Sandhu V, Sood N, Sood A, Malhotra V. Histopathological analysis of chronic gastritis and correlation of pathological features with each other and with endoscopic findings. Pol J Pathol 2012; 63(3):172-8.
3. World gastroenterology organization global guideline: Helicobacter pylori in developing countries. J Diagn Dis 2011;12(5):319-26.
4. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis.The updated Sydney System.International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996:20(10):1161-81.
5. Odze RD, Goldblum JR. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. In: Richard H. Lash , Gregory Y,Lauwers Robert D,Odze , Robert M. Genta, editors. Inflammatory Disorders of the Stomach.2nd ed. Elsevier Health Sciences; 2009. p.269-320.
6. Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102(8):1808-25.
7. el-Zimaity HM. Accurate diagnosis of Helicobacter pylori with biopsy. Gastroenterol Clin North Am 2000;29(4):863-9.
8. Lawson AJ, Elviss NC, Owen RJ. Real-time PCR detection and frequency of 16S rDNA mutations associated with resistance and reduced susceptibility to tetracycline in Helicobacter pylori from England and Wales. J Antimicrob Chemother 2005; 56(2):282-6.
9. Ho B, Marshall BJ. Accurate diagnosis of Helicobacter pylori: serologic testing. Gastroenterol Clin North Am 2000;29(4):853-62.
10. Gisbert JP, Pajares JM. Review article: 13C?urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol Ther 2004;20(10):1001-17.
11.Wang XI, Zhang S, Abreo F, Thomas J. The role of routine immunohistochemistry for Helicobacter pylori in a gastric biopsy. Ann diagn Pathol 2010;14(4):256-9.
12. Wabinga HR. Comparison of immunohistochemical and modified Giemsastain for demonstration of Helicobacter pylori infection in an African population. African Health Sci 2002;2(2):52-5.
13. Haqqani MT, Langdale-Brown B. Campylobacter pylori--acridine orange stain and ultraviolet fluorescence. Histopathology 1988;12(4):456-457.
14. KanimozhiGowsik, Archana V. Comparison of various histochemical staining methods for identification of Helicobacter pylori. Trop J Path Microb 2019;5(9): 692-695.
15. Yakoob J, Jafri W, Abid S, Jafri N, Abbas Z, Hamid S, et al. Role of rapid urease test and histopathology in the diagnosis of Helicobacter pylori infection in a developing country. BMC Gastroenterol 2005; 5:38.
16. Kaur G, Madhavan M, Basri AH, Sain AH, Hussain MS, Yatiban MK, Naing NN. Rapid diagnosis ofHelicobacter pylori infection in gastric imprint smears. Southeast Asian J Trop Med Public Health 2004;35(3):676-680.
17. Mégraud F, Lehours P. Helicobacter pylori Detection and Antimicrobial Susceptibility Testing. Clin Microbiol Rev 2007;20:280-322.
18. Ahmad F, Jaffar R, Khan I. Helicobacter pylori detection in chronic gastritis: a comparison of staining methods. J Ayub Med Coll Abbottabad 2011; 23(2):112-4.
19. Sulakshana MS, Siddiq M. Ahmed and Raghupathi A R. A histopathological study of the association of Helicobacter pylori with gastric Malignancies. Int J Curr Aca Rev 2015;3(3):10-28.
20. Ashton M, Diss T C, Isaacson P G.Detection of H.pylori in gastric biopsy and resection specimens. J Clin Pathol 1996;49:107-111.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License