IJCRR - 13(8), April, 2021
Pages: 58-61
Date of Publication: 25-Apr-2021
Print Article
Download XML Download PDF
A Study of Complications Arising Due to Peripherally Inserted Central Catheters in Oncological Settings: Single Centre Experience in North India
Author: Javid Muzamil, Imran Nazir Salroo, Showkat Shah, Musharaf Bashir
Category: Healthcare
Abstract:Background: In a haemato-oncological setting, a long term drug delivery route is very much needed. Peripherally inserted central catheters (PICC) are used for this purpose. Objective: This study was aimed to observe the complications associated with PICC insertion. Methods: This was a prospective observational study over one year in the Department of Medical Oncology at Khyber Superspecialty hospital, Srinagar, India. A total of 200 subjects participated in this study. The data was collected concerning disease, catheter dwell time, and complications. USG guided or unguided PICCs were inserted. The position of PICC was confirmed. PICC line was then fixed with stat lock. Complications associated with this procedure were noted. Out of 200 patients enrolled, 60% (120/200) were males and 40% (80/200) were females. Unguided PICC was inserted in 90% and USG guided in 10% of participants. Unguided procedures were done in 2 minutes with an average blood loss of 2 ml. Results: Around 5% reported pain within 24 hours and 2.5% reported fever beyond 24 hours. The average dwell time for a single PICC was 5 months. 35% developed line fracture which was correctable. Only 3% developed serious complications viz; CABSI (Catheter-associated bloodstream infections) in 2.5% and thrombosis in 0.5%. Conclusion: PICCs are safe and can be used for extended periods. Unguided PICCs were placed in most of the participants without any major complications. From this study, it appears that PICCs are safe to use with low thrombotic and infectious complications. The most common complications were line fracture, pain and fever.
Keywords: PICC, USG, CABSI, CVC, Catheter, Thrombosis
Full Text:
Introduction
Prolonged treatment and frequent administration of chemotherapeutic drugs, blood and blood products with supportive care is the cornerstone of cancer patient care.1 For this purpose, central venous access devices (CVAD) such as Chemo-port (CP) and peripherally inserted central catheters (PICC) are being used.2 Central venous catheters (CVC) were introduced in the 1980s and they are in use since then.3 The cost of these devices and maintenance is a crucial factor, especially in low-income countries.4,5 This study was aimed to assess the advantages and complications involved with PICC over one year.
Material and Methods
After getting the clearance from Institutional Ethics Committee this prospective study was done at Khyber super speciality hospital, Srinagar, J&K, India. For this study, we enrolled 200 patients who required chemotherapy beyond 4 months. In this study males were more in number than females with a male to female ratio of 1.5:1. Only PICCs with a one-way valve [Bard 4F Groshong] were used. After all antiseptic precautions, USG guided or unguided PICCs were inserted depending upon visualization of upper arm veins. All PICCs were put after cleaning the local area and after giving 0.5ml 2% lignocaine. The position of PICC was confirmed and the length was adjusted at the angle of Louis with the use of a guidewire. Normal saline [10 ml] flush was used and the patient was asked to report any hissing sound or cold sensation from the ipsilateral ear, which was confirmed by x-ray chest. PICC line was then fixed with stat lock. The long-term complications associated with the procedure were noted. The average time for the procedure varied between guided and unguided. It was 2 min in unguided and 10 min in USG guided.
Results
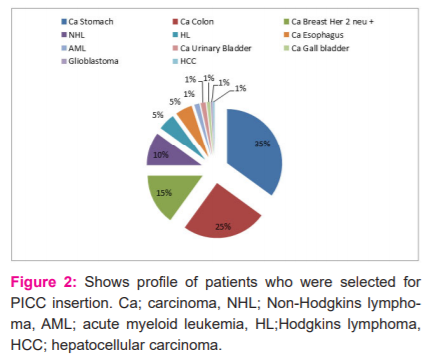
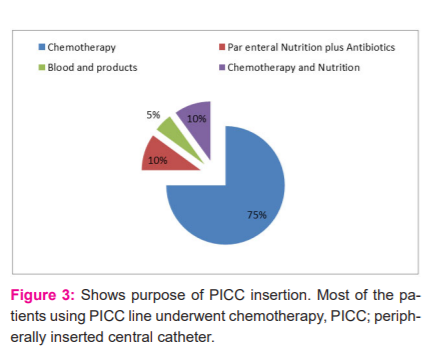
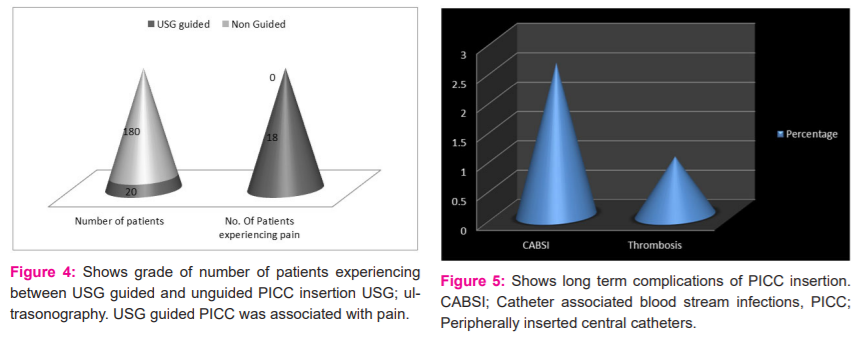
Depending upon the access of veins, USG guided or unguided PICC was inserted. Average blood loss was more in USG guided PICC insertion as shown in Table 1. USG guided insertion of PICC is shown in Figure 1. The disease profile of patients in this study is shown in Figure 2. Most patients had carcinoma stomach followed by carcinoma colon. PICCs were used for various purposes such as prolonged chemotherapy, parenteral nutrition, blood transfusion and for delivering fluids and antibiotics in infections as shown in Figure 3. In this study, it was observed that around 90% of USG guided PICC insertions perceived some degree of pain as shown in Figure 4. Post-procedure 24-hour complications were either pain or thrombophlebitis. Local site pain was reported by 5% of patients and thrombophlebitis in 7.5%. No patient reported fever within 24 hours. Only 2.5% of patients reported fever after 24 hours within 1 week, which was uncomplicated. Delayed complications (beyond 4 months) occurred in few patients, which needed premature removal as shown in Figure 5.
Discussion
In an Intensive Care Unit (ICU) placement of CVC is the most common procedure to be performed. The safety of this procedure has been enhanced by the addition of ultrasonographic guidance.6 Literature shows that USG (ultrasonography) guided CVC leads to lesser failed attempts and complications.7 PICCs provide long-term intravenous access for delivering antibiotics, blood products, chemotherapy and nutrition.7 PICCs have gained popularity as it has lesser risks than CVCs.8 In a prospective study, Gowardman et al.9 studied 410 critically ill patients who required CVC placement. They compared colonization and CABSI rates at various sites where CVC was placed and they observed that colonization was higher at the internal jugular [HR 3.64; 95% CI 1.32-10.00; p=0.01] and femoral [HR 5.15; 95% CI 1.82-14.51; p=0.004] sites than at the subclavian vein. They also observed that CVCs were less colonized in females than in males [HR 0.49; 95% CI 0.26-0.89; p=0.02]. They concluded that colonization was influenced by the site of placement of PICCs and gender. Norwood et al.10 conducted a prospective observational study in trauma patients who received CVCs to determine the rates of CABSI. They observed that the rates of CABSI were 5% [5/1000 catheter days] and 1.5% [1.51/1000 catheter days], respectively. Moreover, subclavian PICCs were in place longer than femoral or internal jugular PICCs (p<0001), but the colonization rate was significantly lower (p =0.03; relative risk, 0.34; 95% confidence interval, 0.15-0.77) in subclavian PICCs. They did not find any difference in CABSI rates among PICCs placed at different anatomical sites. They concluded that subclavian PICCs developed colonization in the end. Rates of CABSI ranged from 7% to 60% in different studies by Kim HJ et al.11 and Babu KG et al.2 In this study the rate of CABSI was around 2% only. In a study by Jain et al.3, it was seen that the median PICC dwells time was 59 days whereas in the current study it was 150 days. In this study, Bard Groshong, PICC lines were used in 200 patients. Difficulty in insertion was noted in 1% of the patient against 5% cases of Certofix and 12.5% cases of Cavafix by Kumar et al.12 Extravasation was noted in 3.89%, DVT in 2.78% and thrombophlebitis in 1.11% cases in Babu KG et al.2 whereas in this study no extravasation was reported, thrombophlebitis was seen in 7.5% patients and thrombosis in 0.5%. We did not witness any catheter fragment dislodgement contrary to Babu KG et al.2 Organisms recovered were gram-positive in 60%, which is the same as in study reported by Kumar et al.12 Premature removal of PICC was done in 2.5% in our study due to complications whereas it was 27.3% in a study by Babu KG et al.2 In this study, PICCs served the purpose in almost all patients with minimal complications.
Conclusion
PICC are a very important part of cancer patient management. This is the first study of its kind with almost no complication rate. In this study PICC [Bard Groshong 4F] one-way lines were used instead of Cavafix. From this study, it may be concluded that PICCs [Bard Groshong 4F] with one-way valve is associated with the least complications and could substitute the chemo Port implant which is invasive, costly and difficult to place.
Acknowledgements: We acknowledge the great help received from the researchers whose articles are cited and included in references to this manuscript.
Conflict of interest: None
Funding: None.
References:
-
Jordan K, Feyer P, Holler U, Link H, Wormann B, Jahn F. Supportive Treatments for Patients with Cancer. Dtsch Arztebl Int 2017;114(28): 481-87.
-
Babu KG, Babu MCS, Lokanatha D, Bhat GR. Outcomes, cost comparison, and patient satisfaction during long-term central venous access in cancer patients: Experience from a Tertiary Care Cancer Institute in South India. Indian J Med Paediatr Oncol 2016; 47(4):232-38.
-
Jain SA, Shukla SN, Talati SS, Parikh SK, Bhat SJ, Maka V.A retrospective study of central venous catheters GCRI experience. Indian J Med Paediatr Oncol 2013; 34(4): 238-41.
-
Cai Y, Zhu M, Sun W, Cao X, Wu H. Study on the cost attributable to central venous catheter-related bloodstream infection and its influencing factors in a tertiary hospital in China. Health Qual Life Outcomes 2018;16:198.
-
Gonzalez R, Cassaro S. Percutaneous Central Catheter (PICC). Treasure Island (FL); Stat Pearl Publishing: 2020 Jan.
-
Baviskar AS, Khatib KI, Bhoi S, Galwankar SC, Dongare HC.Confirmation of endovenous placement of central catheter using the ultrasonographic “bubble test”. Indian J Crit Care Med 2015;19(1):38-41.
-
Leung J, Duffy M, Finckh A. Real-time ultrasonographically-guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: A randomized, prospective study. Ann Emerg Med 2006; 48:540–47.
-
Smith RN, Nolan JP. Central venous catheters. BMJ 2013; 347: f6570.
-
Gowardman JR, Robertson IK, Parkes S, Rickard CM. Influence of insertion site on central venous catheter colonisation and bloodstream infection rates. Intensive Care Med 2008; 34:1038-45.
-
Norwood S, Wilkins HE, Vallina VL. The safety of prolonging the use of central venous catheters: A prospective analysis of the effects of using antiseptic bonded catheters with daily site care. Crit Care Med 2000;28:1376-82.
-
Kim HJ, Yun J. Safety and effectiveness of central venous catheters in patients with cancer: Prospective observational study. J Korean Med Sci 2010;25:1748-53.
-
Kumar AH, Srinivasan NM, Thakkar JM. A prospective observational study of the outcome of central venous catheterization in 100 patients. Anaesth Essays Res 2013; 7:71-5.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License