IJCRR - 13(8), April, 2021
Pages: 16-21
Date of Publication: 25-Apr-2021
Print Article
Download XML Download PDF
Role of Cartridge Based Nucleic Acid Amplification Testing (CBNAAT) in Diagnosis of Extrapulmonary Tuberculosis- Experience from a Teaching Institution in Eastern India
Author: S Mukherjee, D Biswas, S Begum, P Ghosh, A Pal, S Sarkar
Category: Healthcare
Abstract:Background: Diagnosis of extrapulmonary tuberculosis is often challenging due to the lack of a gold standard test. Cartridge based nucleic acid amplification testing (CBNAAT) has been advocated for rapid diagnosis of extra-pulmonary tuberculosis and to identify rifampicin resistance. Objective: To evaluate the diagnostic yield of Cartridge Based Nucleic Acid Amplification Testing (CBNAAT) in different forms of extrapulmonary tuberculosis and to compare its efficacy between different forms of EPTB. Methods: This is hospital-based prospective observational study conducted in the outpatient and indoor Respiratory Medi�cine department. All adult patients of EPTB were recruited for the study and CBNAAT was performed from appropriate clinical specimens in all of them. Results were analyzed in light of the diagnostic yield of CBNAAT with special emphasis on comparing CBNAAT results between different groups of EPTB. Results: Out of 502 cases of EPTB, CBNAAT was positive in 138 (27.5%) patients. The mean age of the study population was 36.49\?14.05 years with 61.1% males. Tubercular meningitis (p-0.023) and tubercular empyema (0.04) were more prevalent in the under 30 years age group compared to other forms of EPTB. Sensitivity of CBNAAT was good for paravertebral abscess, tubercular empyema, tubercular meningitis, cold abscess and tubercular lymphadenitis, but sensitivity was very low in tuber�cular pleural effusion and ascites. CBNAAT was significantly more positive in samples containing pus (70 out of 81, 86.41%; p-< 0.0001). Rifampicin resistance was detected in 14 cases, of which four also showed isoniazid resistance on Line Probe Assay Conclusion: CBNAAT adds significantly to the diagnostic yield of EPTB in comparison to conventional methods, but its sensitiv�ity varies in different forms of extrapulmonary tuberculosis. It has the additional advantage of identifying rifampicin resistance with high sensitivity and specificity.
Keywords: Extrapulmonary tuberculosis, Nucleic acid amplification test, Pus, Rifampicin resistance, Multi-drug resistant tuberculosis
Full Text:
INTRODUCTION
Extrapulmonary tuberculosis (EPTB) accounts for about one-fourth of tuberculosis (TB) cases worldwide and in India, it accounts for about 15%-20% of the total tuberculosis cases.1-3 Diagnosis of EPTB is often challenging because the yield of smear and/or mycobacterial culture is often low and time-consuming. Demonstration of a caseating granuloma on biopsy specimens is not always confirmatory but the facility of histopathology with mycobacterial culture from the biopsy tissue sample is not always available.4-6 Moreover, mycobacterial culture is time-consuming and is costly, too.7,8 CBNAAT is a completely automated test that utilizes principles of nested polymerase chain reaction (PCR)and can identify genes specific for Mycobacterium tuberculosis. It detects rifampicin resistance as well and the result is obtained very quickly, World Health organisation has endorsed the use of CBNAAT as a rapid molecular diagnostic test.9,10 There has been a paucity of data from eastern India regarding the role of CBNAAT in the diagnosis of EPTB cases post-implementation of CBNAAT as a rapid diagnostic test in RNTCP.In this background, the study was conducted to evaluate the diagnostic role of CBNAATin in different forms of EPTB.
MATERIAL AND METHODS
A prospective, observational and analytical study of all adult cases (above 12 years of age) of EPTB, attending outpatients department or admitted in the Respiratory Medicine and other departments of a tertiary care Hospital in Kolkata was undertaken throughout one and half year (July 2017-December 2018) (IECNo:CMSDH/IEC/78/04-2017)
Case definition11
Microbiologically confirmed Extra-Pulmonary tuberculosis (EPTB): refers to a presumptive EPTB with nonrespiratory clinical sample positive for acid-fast bacilli (AFB) by Ziehl-Neelsen (Z-N) stain or positive for Mycobacterium tuberculosis on culture, or positive for tuberculosis through a quality-assured rapid molecular diagnostic test.
Clinically/histologically diagnosed EPTB case
A patient diagnosed as EPTB on clinical, radiological and/or cytopathology /histopathology evidence of caseating epithelioid granuloma with giant cells consistent with tuberculosis in absence of a microbiological confirmation. Written informed consent was obtained. Institutional ethics committee approval was taken (IEC No.- (CMSDH/IEC/78/04-2017).
Inclusion criteria
All cases of microbiologically confirmed or clinically or histologically diagnosed EPTB cases attending outpatients department or admitted in different indoor departments of the teaching hospital during the study period
Exclusion criteria
Patients with age less than 12 years and patients not giving consent for the study were excluded from the study.
Study protocol
All patients satisfying the case definition and inclusion criteria were considered for subsequent investigation and analysis. Patients were evaluated with history and clinical examination and organ-specific sample were sent for CBNAAT as per Revised National Tuberculosis Control Program (RNTCP) protocol for Mycobacterium tuberculosis(Cepheid, GX-IV Processing Unit: 11.00" w x 12.00" h x 11.70" d, GXIV-4-D).9,11,12 The second falcon tube was sent for Line Probe Assay (LPA) for first-line and second-line baseline drug sensitivity testing (FL-LPA and SL-LPA) to the intermediate reference laboratory (IRL) if Rifampicin resistance detected. Clinical specimens were also sent for smear microscopy for AFB by Z-N stain. Clinical samples were tested for mycobacterial culture (BACTEC), cytopathology, histopathology, cytology, biochemical estimations, estimation of adenosine deaminase (ADA) in relevant cases. Sputum for AFB smear and chest X-ray posteroanterior (PA) view was done in all patients. Computed Tomography (CECT) scan of thorax with contrast, ultrasonography (USG) of the abdomen, magnetic resonance imaging (MRI) of brain and spine were done additionally in respective cases of EPTB. Blood was sent for testing for HIV infection at the integrated counselling and testing centre (ICTC) of our hospital. Relevant haematological investigations like fasting blood sugar, complete hemogram, urea, creatinine and baseline liver function test were also done in all patients.
A composite reference system (CRS) [ comprising of positive AFB smear and/or positive mycobacterial culture and/or positive cytopathology/histopathology demonstrating caseating epithelioid granuloma with giant cells, and/or positive radiological findings, and/or positive cellular and biochemical parameters (lymphocytosis and raised ADA ), and/or clinical diagnosis of EPTB with response to treatment with antitubercular drugs] was considered as the gold standard for diagnosis of EPTB in this study and performance of CBNAAT was compared with the CRS.
Statistical analysis:
SPSS version 20.0 (SPSS inc. Chicago, IL) was used for statistical analysis. Categorical data were expressed in terms of percentages and mean±standard deviation (mean±SD) was used for analyzing continuous variables. Fisher’s exact test and Chi-Square test were used for calculation of P-value and P value of less than 0.05 was considered to be of significance for this study.
RESULTS
Distribution of Extra-pulmonary TB cases
A total of 502 cases of EPTB were recruited during the study period. Out of this 502 cases, 284 were tubercular pleural effusion, ten were tubercular empyema, 26 cases were tubercular ascites, 114 cases were tubercular lymphadenopathy, 51 cases were tubercular cold abscess, eleven cases were caries spine with paravertebral abscess, four cases were tubercular meningitis and two cases were endometrial tuberculosis. Twenty-five cases (4.98%) had a previous history of anti-tubercular drug intake for more than one month. Seventy-three (14.5%) patients were referred from private practitioners.
Demographic profile
The mean age of EPTB patients in the study population was 36.49 ±14.05 years (mean±SD) with male predominance (61.1%, 306 of 502). There was no significant difference in age distribution between male and female EPTB patients but tubercular meningitis (p-0.023) and tubercular empyema (p-0.04) affected the younger population more compared to other subgroups of EPTB. Disseminated tuberculosis was found in five cases and was significantly more associated with TB meningitis (p-0.003). Diabetes mellitus was detected in 36 patients (7.17%) and human immunodeficiency virus (HIV) co-infection was found in ten cases (1.99%).
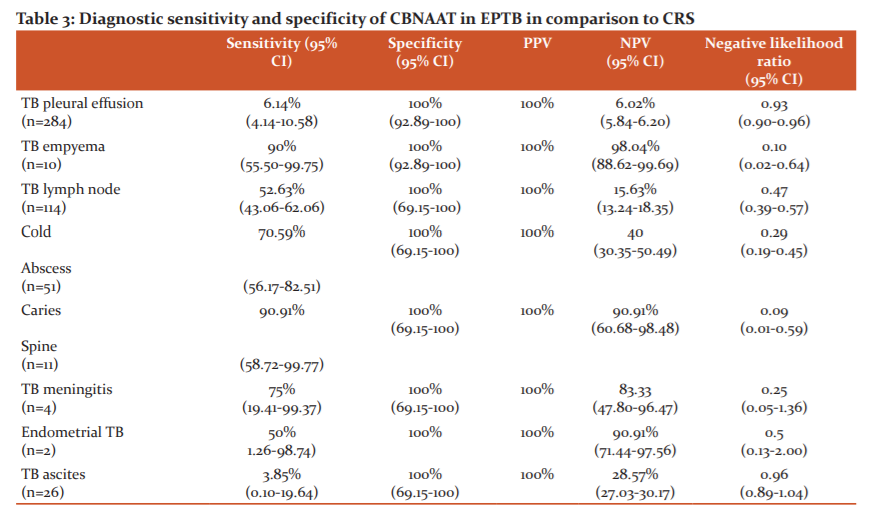
The pleural fluid AFB smear had a yield of 4.8% (12 out of 294) (tubercular effusion 5 out of 284; Tubercular empyema 7 out of 10). Sputum for AFB smear was positive in four out of ten (40%) empyema cases. Pleural fluid ADA level was more than 70 unit in 129 out of 284 patients (47.4%). Caseating granuloma was detected in 18 out of 35 pleural biopsy specimens (51%). Lymph node FNAC showed AFB smear positivity in 28 out of 114 cases (24.6%), and five out of eleven (45.5%) cases of cold abscess aspirate.
CBNAAT
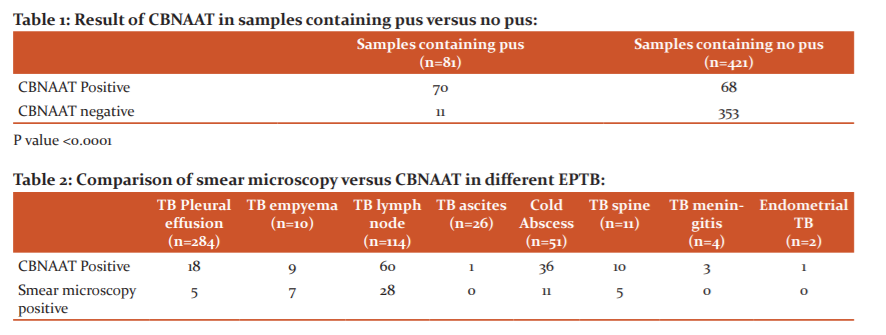
Overall, CBNAAT was positive in 138 patients out of 502 patients (27.5%). CBNAAT result showed very high yield in caries spine (10 out of 11; 90.9%), tubercular empyema (9 out of 10; 90%), TB meningitis (3 out of 4; 75%) and tubercular cold abscess (36 out of 51; 74.5%); the moderate yield was seen in tubercular lymphadenopathy (60 out of 114; 52.6%) and endometrial tuberculosis (50%); but in case of tubercular pleural effusion (6.3%) and ascites (3.8%) yield of CBNAAT was very low. On comparing the rate of CBNAAT positivity among different organ-specific samples, the yield was very highly positive in samples containing pus (p-<0.0001) [Table 1]. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and negative likelihood ratio of CBNAAT for pus samples were 86.4% (95% CI-69.28%-96.24%), 100% (95% CI- 92.89%-100%),100%, 92.59% (95% CI- 83.39%-96.89% ) and 0.13 ( 95% CI-0.05-0.33), when compared to CRS.
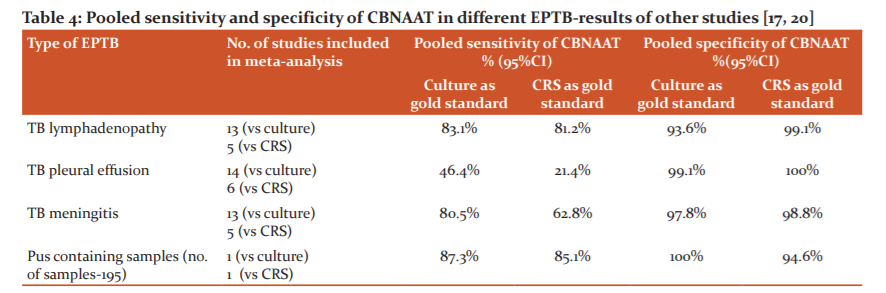
The use of CBNAAT improved the chances of microbiologically confirmed EPTB diagnosis in 82 out 502 (16.3%) cases when compared to smear positivity only [Table 2]. When CBNAAT result was compared to a CRS as the gold standard, its sensitivity and specificity varied among different EPTB subgroups- sensitivity was found very high for caries spine (90.91%), TB empyema (90%), TB meningitis (75%), and cold abscess (70.59%%); whereas it was very low for tubercular pleural effusion (6.14%) and ascites(3.85%%) [Table 3].
Rifampicin resistance (RR) was detected in 14 cases of EPTB (2.79%), of these seven cases of RR-TB was found in TB lymphadenopathy, five RR-TB was found among cases of tubercular pleural effusion and two cases of tubercular cold abscess were RR-TB. Five of these 14 (35.71%) RR-TB cases were patients of initial drug-resistant TB. Sputum for CBNAAT also showed rifampicin resistance in two patients of pleural effusion with RR-TB. On-Line probe assay (LPA) resistance to isoniazid was also documented in four of these 14 (28.57%) RR-TB patients but second-line LPA (SL LPA) did not show any additional drug resistance.
DISCUSSION
India accounts for around one-fourth of the global tuberculosis cases.10,11,13 Confirmation of diagnosis of EPTB often poses challenges to clinicians as microbiological confirmation of disease due to paucibacillary nature of the disease.4,6 The yield of biopsy and histopathological confirmation of diagnosis varies widely ranging from 50%-97% depending on the technical skill and accessibility.12,6,14 WHO has recommended CBNAAT as a rapid diagnostic test for diagnosis of tuberculosis especially drug-resistant tuberculosis, paediatric tuberculosis, TB-HIV co-infection, extrapulmonary tuberculosis and sputum smear-negative PTB.9,11 Moreover it gives result within two hours and can identify rifampicin resistance at the same time.9,10,12
Tubercular pleural effusion was the commonest form of EPTB in this study, being found in 58.17% of cases, followed by tubercular lymphadenopathy (22.71%). In this study, the mean age of EPTB patients was 36.49 ±14.05 years (mean±SD). Tubercular meningitis and tubercular empyema occurred in the younger age group with a mean age of 20.33±8.08 years and 27.4±10.18 years respectively, which was quite similar to observations by Kundu et al (mean age-32.7 years)[15] and Acharya P et al. (commonest age group-20-40 years).16
The overall sensitivity of CBNAAT was low (27.5%) in this study compared to most of the published studies in the literature but its sensitivity varied greatly among different forms of EPTB. Tortoli et al.,17 Vadwai et al.,4 Lighthelm et al.,18 and Causse et al. 19 have shown a high sensitivity of CBNAAT of 81.3% (95% CI-76.2–85.8), 80.6% (CI-75.5–85.0), 96.6% (CI-86.6–100) and 95.1 (83.5–99.4)respectively in their studies. Two of the four studies used a composite reference standard as the gold standard, while the other two (Causse et al. and Lighthelm et al.) adapted culture as the gold standard. On the other hand, Friedrich et al.20 and Moure et al.21 have described much lower sensitivities of 25% (CI-8.7–49.1) and 58.3% (CI-48.5–67.8) respectively in their studies. Specificity was very high in all of the studies, ranging from 88.9% to 100% and this was in line with the 100% specificity of CBNAAT in our study.17-21 Overall sensitivity of CBNAAT in EPTB depends on several factors like the relative proportion of different types of EPTB, sample selection, diagnostic criteria, selection of diagnostic gold standard, sample processing, etc. The reference standard for calculating the sensitivity of CBNAAT was different for different studies and this can explain the variation in sensitivity among different studies. However, formulation of composite reference standard comprising of multiple parameters as the diagnostic gold standard for comparison of CBNAAT performance is better as because, in isolation, neither mycobacterial culture nor histopathology can be taken as the gold standard for diagnosis of EPTB. Hence, comparing CBNAAT sensitivity against culture or histopathology alone as the gold standard may overestimate pooled sensitivity of CBNAAT. It has been shown that pooled sensitivity of CBNAAT become lower when a composite reference standard has taken as the gold standard in comparison to mycobacterial culture as the gold standard.22,23 In our study, 61.75% (310 out of 502) of cases were cases of tubercular pleural effusion and ascites, where pooled sensitivity of CBNAAT is very low in the published literature22, on the other hand, a study by Tortoli et al.17 had only 24% pleural effusion cases in their study population, Causse et al.19 had only 19.4% of samples containing pleural or other body fluids, and Lighthelm LJ et al.18 only looked at lymph node samples. Moreover, a composite reference standard was taken as the gold standard for diagnosis of tuberculosis in this study population, in contrast to culture being taken as the gold standard by several studies.18,19 We also could not include lymph node and other tissue biopsy samples in our study due to lack of histology sample processing facility in our CBNAAT site These factors were probably responsible for low overall sensitivity of CBNAAT in EPTB in this study in comparison to most other studies.
Analysis of the performance of CBNAAT for individual forms of EPTB in this study revealed that CBNAAT sensitivity was very high in caries spine (90.91%), tubercular empyema (90%), and cold abscess (70.59%); in tubercular lymphadenopathy (52.63%) and endometrial tuberculosis (50%) CBNAAT was moderately sensitive; but, on the other hand, sensitivity of CBNAAT was very low for tubercular pleural effusion (6.14%) and tubercular ascites (3.85%). There was a striking difference in CBNAAT sensitivity between tubercular empyema and tubercular pleural effusion (90% versus 6.14%). This may be explained by the difference in pathogenesis between tubercular empyema and tubercular pleural effusion, while the former has a high bacillary load, the latter entity is paucibacillary.24,25 CBNAAT was found to be highly sensitive on specimens containing pus (70 out of 81; sensitivity 86.4%, specificity 100%). This finding has been supported by the study of Tortoli et al.17, who have also found a sensitivity of more than 85% in purulent samples. Pooled sensitivity and specificity of CBNAAT for different forms of EPTB by different studies have been shown in Table 4, and it shows that results of CBNAAT sensitivity for individual forms of EPTB in this study were following the literature published. 17, 22 Very low yields of CBNAAT in pleural fluid and ascitic fluid in our study have also been supported by the study of Pravin KN et al.26 who have demonstrated CBNAAT positivity in 10% and 9.3% respectively from pleural fluid and peritoneal fluid in their study. The results vary widely depending on the reference standard adapted for analysis. The pooled sensitivity of CBNAAT in pleural fluid was also found to be low (21.4%; 95% CI 8.8 – 33.9) in a meta-analysis report.22 Due to the very low sensitivity of CBNAAT in pleural effusion, the Index TB guideline for EPTB has recommended against routine use of CBNAAT in the diagnosis of tubercular pleural effusion.3
In this study, the use of CBNAAT had prompted an increase in the diagnosis of microbiologically confirmed EPTB by 15.94% in comparison to AFB smear microscopy. The result was more marked in the case of tubercular meningitis (75%), tubercular cold abscess (49.09%), caries spine with paravertebral abscess (45.45%), endometrial tuberculosis (50%), tubercular lymphadenopathy (28.07%), tubercular empyema (20%) -in all these forms of EPTB, confirmation of a microbiological diagnosis is difficult in most cases.4-6
Rifampicin resistance was detected in 14 cases of EPTB (2.9%) in this study. In a study by Tadesse M et al from Ethiopia, it has also been shown that sensitivity and specificity of CBNAAT were 87.8% (95% CI: 81.0-94.5) and 91.1% (95% CI: 82.8-99.4) respectively against a CRS in fine-needle aspiration (FNA) samples from presumptive tubercular lymphadenopathy cases, and rifampicin resistance was noted in four cases (4.7%). 27 In our study, rifampicin resistance was seen in 6.14% cases of tubercular lymphadenopathy, in 1.70% cases of tubercular pleural effusion and 3.9% cases of the tubercular cold abscess. Other studies have also demonstrated similar evidence of rifampicin resistance by CBNAAT in cases of EPTB.4,17,21,26 These findings have put a question mark over the common notion that EPTB is mostly paucibacillary and the possibility of drug resistance is extremely rare in EPTB.
The limitation of the study was that we did not compare CBNAAT with mycobacterial culture and histopathology as mycobacterial culture and histopathology were not done in all cases of EPTB in this study. This study could not compare the yield of CBNAAT between HIV seropositive and seronegative patients. Future studies in this direction are needed.
CONCLUSION
Sensitivity of CBNAAT varied among different forms of EPTB. CBNAAT was moderate to highly sensitive in paravertebral abscess, cold abscess, lymph node TB, TB meningitis and endometrial tuberculosis and is recommended strongly for diagnosis of these forms of EPTB. The diagnostic role of CBNAAT is unclear in cases of tubercular pleural effusion and ascites due to very low sensitivity. Hence routine use of CBNAAT in the diagnosis of tubercular pleural effusion and ascites is not beneficial and cost-effective especially in resource-limited countries like India and other third world countries with a high tuberculosis burden. Keeping in mind its rapidity in diagnosis as well as identification of rifampicin resistance, CBNAAT should routinely be tested in all forms of EPTB except tubercular pleural effusion and ascites especially in developing nations with a high burden of tuberculosis like India.
Acknowledgements: 1. Prof. Susmita Bhattacharya, Head, Department of Microbiology, College of Medicine and Sagar Dutta Hospital; 2. Mr. Chandan Mondal, Senior Treatment and Laboratory, Supervisor, RNTCP, College of Medicine and Sagar Dutta Hospital; 3. Mr. SankaracharyyaHalder, Department of Microbiology, College of Medicine and Sagar Dutta Hospital
Conflict of interest- None
Source of funding – None
References:
-
World Health Organization. Global tuberculosis report 2016. Geneva: WHO;2016. Available from:
http://apps.who.int/iris/bitstream/10665/137094/1/9789241565394_eng.pdf?ua=1
-
Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res 2004;120:316-53.
-
Sharma SK, Ryan H, Khaparde S, Sachdeva KS, Singh AD, Mohan A, et al. Index-TB guideline. Guidelines of extra-pulmonary tuberculosis for India. Indian J Med Res 2017;145:448-6
-
Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigue C. Xpert MTB/RIF: a New Pillar in Diagnosis of Extrapulmonary Tuberculosis? J Clin Microbiol. 2011;49:2540-5.
-
Halder S, Bose M, Chakrabarti P, Daginawala HF, Harinath BC, Kashyap RS, et al.Improved laboratory diagnosis of tuberculosis-The Indian experience. Tuberculosis 2011;91:414-26.
-
Chakravorty S, Sen MK, Tyagi JS. Diagnosis of extrapulmonary tuberculosis by smear, culture and PCR using universal sample processing technology. J Clin Microbiol 2005; 43:4357-62.
-
Moore DF, Guzman JA, Mikhail LT. Reduction in turnaround time for laboratory diagnosis of pulmonary tuberculosis by routine use of a nucleic acid amplification test. Diagn Microbiol Infect Dis 2005;52:247–254.
-
Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: part II. Active tuberculosis and drug resistance. Expert Rev Mol Diagn 2006;6:423–432.
-
Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. Geneva, World Health Organization, 2013. http://www.who.int/tb/laboratory/policy_statements/en/)
-
Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol 2011;6:1067–1082.
-
Central TB Division(India).Revised National TB Control Programme. Technical and Operational Guidelines for Tuberculosis Control in India 2016.
-
Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K . Rapid Detection of Mycobacterium Tuberculosis and Rifampin Resistance by Use of On-Demand, Near-Patient Technology. J Clin Microbiol 2010;48: 229–37.
-
Annual Status Report.Central TB Division. Official website of the Revised National TB Control Programme, Directorate General of Health Services, Ministry of Health & Family Welfare Government of India. 2015. [accessed on March 25, 2017]. Available from: http://www.tbcindia.org.
-
Aggarwal AN, Gupta D, Jindal SK. Diagnosis of tubercular pleural effusion. Indian J. Chest Dis Allied Sci 1999;41:89-100.
-
Kundu S, Mitra S, Mukherjee S, Das S. Adult Thoracic empyema: A comparative analysis of tuberculous and non-tuberculous aetiology in 75 patients.
Lung Ind 2010;27:196-201.
-
Acharya PR, Shah KV. Empyema thoracis: A clinical study. Ann Thorac Med 2007; 2:14-17.
-
Tortoli E, Russo C, Piersimoni C, Mazzola E, Monte PD, Pascarella M, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J 2012;40:442-447.
-
Ligthelm LJ, Nicol MP, Hoek KG, Jacobson R, van Helden PD, Marais BJ, et al. Xpert MTB/RIF for rapid diagnosis of tuberculous lymphadenitis from fine-needle-aspiration biopsy specimens. J Clin Microbiol 2011;49:3967-3970.
-
Causse M, Ruiz P, Juan Bautista GA, Casal M. Comparison of two molecular methods for the rapid diagnosis of extrapulmonary tuberculosis. J Clin Microbiol 2011:49(8):3065-3067.
-
Friedrich SO, von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol 2011; 49:4341–4342.
-
Moure R, Munoz L, Torres M, Santin M, Martin R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol 2011;49:1137–9.
-
Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR.Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 2014;44:435-46.
-
Sehgal IS, Dhooria S, Aggarwal AN, Behera D, Agarwal R.Diagnostic Performance of Xpert MTB/RIF in Tuberculous Pleural Effusion: Systematic Review and Meta-analysis. J Clin Microbiol 2016;54:1133-6.
-
Sowjanya DS, Behera G, Reddy VVR, Praveen JV. CBNAAT: a Novel Diagnostic Tool For Rapid And Specific Detection Of Mycobacterium Tuberculosis In Pulmonary Samples. Int J Health Res Integ Med Sci 2014;1:28-31.
-
Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005-15.
-
Pravin KN and Chourasia E. Use of GeneXpert Assay for Diagnosis of Tuberculosis From Body Fluid Specimens, a 2 Years Study. J Microbiol Biotechnol 2016; 1(1): 000105.
-
Tadesse M, Abebe G, Abdissa K, Aragaw D, Abdella K, Bekele A, et al. GeneXpert MTB/RIF Assay for the Diagnosis of Tuberculous Lymphadenitis on Concentrated Fine Needle Aspirates in High Tuberculosis Burden Settings. Plos One 2015 ;10(9): e0137471.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License