IJCRR - 2(4), April, 2010
Pages: 20-29
Print Article
Download XML Download PDF
FAST DISPERSING TABLETS OF DICLOFENAC SODIUM WITH NATURAL SUPERDISINTEGRANTS
Author: Wyawahare N.S, Mishra M.U, Bhongade S.L.
Category: Healthcare
Abstract:Objective: In the present study, fast dispersing tablets of Diclofenac Sodium were prepared by using natural super disintegrants obtained from husk and mucilage of seeds of plant Lepidium sativum. Fast dispersing or dissolving tablets are the dosage form which dissolve or disintegrate in oral cavity without the need
of water or chewing. Diclofenac Sodium is a non steroidal anti-inflammatory drug taken to reduce
inflammation and as an analgesic in conditions such as arthritis or acute injury with lower indication of gastrointestinal adverse effects. Method: The husk and mucilage were obtained from the seeds. The
physicochemical properties like density, flow properties, particle size distribution, swelling index and water uptake studies of dried husk and mucilage were evaluated. It was observed that it shows rapid water
uptake and swelling which makes it suitable candidate as disintegrants. Diclofenac Sodium fast dispersing
tablets have been prepared by direct compression method by using mucilage and husk of the seeds of plant Lepidium sativum. Formulation was optimized on the basis of physical properties, swelling time, drug
content, in vitro disintegration and in vitro drug release. The study revealed that husk and mucilage are effective at 4% concentration and showed rapid disintegration of the tablets (25 sec and 22 sec respectively). The husk as well as the mucilage did not interfere with the in vitro release studies. The tablets show almost
complete release within 20 minutes. Conclusion: From this study, it can be concluded that natural disintegrants obtained from Lepidium sativum showed fast disintegrating property.
Keywords: Lepidium sativum, husk and mucilage, fast disintegration
Full Text:
Introduction
Mouth dissolving tablets are the tablets which disperse rapidly in an oral cavity within a minute, without a need of water1 . For these formulations, the small volume of saliva is usually sufficient to result in tablet disintegration in the oral cavity.2 The gediatric and pediatric patients experience difficulty in swallowing tablets which leads to poor patient compliance. Similarly it is difficult to administer the drugs to mentally ill, bed ridden patients or to the patients having difficulty with swallowing the tablets.1 Fast dispersing tablets are a promising approach to overcome above problems. Mouth dissolving tablets breakdown rapidly in small particles without need of water and therefore it is convenient during travelling.2 Apart from patient compliance, rapid onset of action and increase bioavailability are the added advantage in designing of the tablets.3 Fast dissolving drug delivery (FDDTs,) can be achieved by various conventional methods like direct compression, wet granulation, moulding, spray drying, freeze drying and sublimation. In order to allow fast dissolving tablets to dissolve in the mouth, they are made of either very porous and soft- moulded matrices or compressed into tablets with very low compression force, which makes the tablets friable and/or brittle, which are difficult to handle, often requiring specialized peel-off blister packaging.4 Diclofenac is a non-steroidal antiinflammatory drug (NSAID) taken to reduce Inflammation and as an analgesic reducing pain in conditions such as arthritis or acute injury. It can also be used to reduce menstrual pain, dysmenorrhea. The name is derived from its chemical name: 2-(2,6- dichloranilino)phenylacetic acid. Mucilage and gum have been known since long time as a medicinal agent. Nowadays these gums and mucilage is gaining importance in pharmaceutical industries as thickener, suspending and emulsifying agents, binder and film former. As these are vegetative in origin hence the demand of these substances is increasing and therefore new sources been searched.5,6
Material and methods
Diclofenac sodium (Panacea Biotech), microcrystalline cellulose PH 102 (NB Entrepreneurs), Sucrose (Loba chemicals), Talc (Burgoyne Lab and Co), magnesium stearate (Samar chemicals), Potassium dihydrogen orthophosphate (Krypton chemicals)
Method
Isolation of husk2
The seeds of the Lepidium sativum were powdered by automatic grinder and sieved through sieve #80. The seed powder was treated with chloroform. The husk was settled down in separating funnel remaining all the constituents of the seed floats at the top of the liquid. The husk was separated and dried at 400C till completely dried. The dried husk was pulverized by automatic grinder and passed through # 80 sieve.
Isolation of mucilage5,6
The seed of Lepidium sativum contains the mucilage in the outer covering of the seed. The mucilage is enmeshed in the hard covering of the seed. The seeds of the Lepidium sativum swell rapidly but it did not separate from the surface of the seed and forms the thick covering around it which is difficult to remove by general mentioned methods. The seeds were soaked in water for 12 hrs in distilled water. The swollen material was transferred to the blender and blended for 10 min. The mass was then passed through eight fold of muslin cloth.
The filtrate was collected. To the filtrate acetone was added in ratio 1:1, the mucilage was precipitated out. The precipitate was separated using separating funnel. The mucilage is dried in hot air oven at 400C till mucilage completely dried. The dried mass was pulverized using automatic blender and passed through # 80 seive.
Characterization of
Superdisintegrants2,5,6
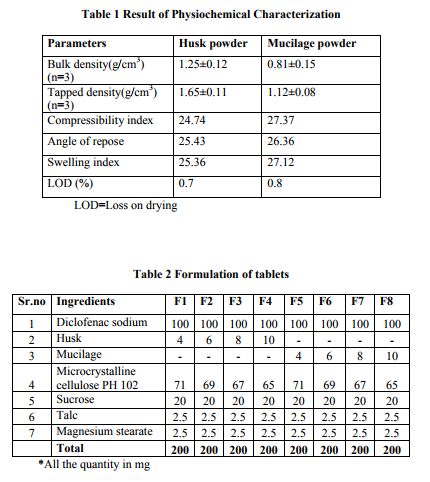
The Superdisintegrants were evaluated for their physiochemical properties such as swelling index, loss on drying, flow properties, density and compatibility. Swelling index: The swelling index is the volume in ml occupied by 1 g of drug. The swelling index of Lepidium sativum seed powder and mucilage were determined according to BP method. 1g of each disintegrants was taken in separate graduated cylinder of 25 ml. To this 25 ml of water was added and shaken vigorously in every 10 min for 4 hrs. The volume occupied by swollen mucilage was measured. The test was carried out in duplicate and swelling index was calculated. The swelling index is tabulated in table no 1.
Loss on drying: The moisture presents in the husk or mucilage influence the stability of the product. High moisture content contributes to the degradation of moisture sensitive drug as well as favor microbial contamination. The moisture content was determined by LOD method. 1g of sample was heated at 1050C in muffle furnace until constant weight was achieved. The %LOD was calculated and tabulated in table no 1.
Density, compressibility and flow property:
Bulk density was determined by taking accurate weight of sample in 100 ml graduated cylinder and volume was measured. For tapped density, cylinder was tapped until the powder bed volume achieves the constant reading. Compressibility index is a measure of ability of solid to get compressed. Flow property is determined in order to determine the effect of the disintegrant on the flow of the blend. Angle of repose is used to determine the flow property of the powder. It is determine by fixed height funnel method. The data is tabulated in table no 1.
Compatibility studies: The drug and disintegrant were mixed properly in ratio 1:0.2. The physical mixtures were studies after 7 days.
Preparation of the tablets
Fast dispersing tablet of Diclofenac sodium 100 mg were prepared by direct compression method. The various ratios of drug and excipients were studied as shown in table no 2. The drug, disintegrant (husk or mucilage), microcrystalline cellulose and sucrose were pass through # 40 sieve. All the ingredients were mixed uniformly in a polybag. Talc and Magnesium stearate were passes through # 60 sieves and added to the polybag and mixed properly. The blend was compressed on a 12 station rotary punch tableting machine using 8 mm of concave punch set.
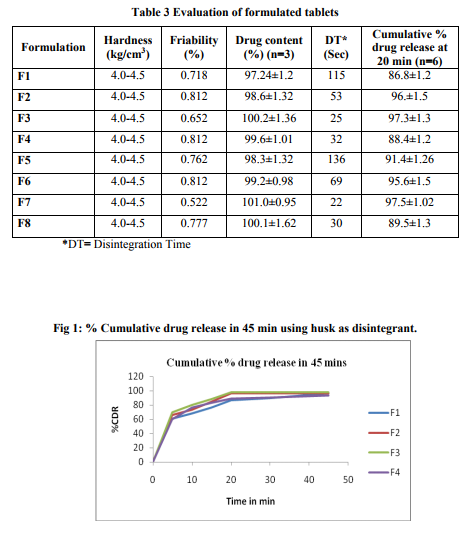
Evaluation of tablets Tablets were evaluated for weight variation, hardness, friability, thickness and in-vitro disintegration time and in-vitro dissolution of the tablets. In weight variation test 20 tablets were randomly selected and average weight was determined using electronic balance. Tablets were weighed individually and compared with average weight and % variation was determined. The hardness of the tablet was determined using Monsanto type hardness tester and the applied pressure for crushing the tablets was determined. Roche frabilator was used to determine%friability of the tablets which was rotated at 25 rpm for 4 min. % friability = 100 x Wo-Wt/Wo. Drug content study of tablets was studied by method as specified in IP 1996. The data is tabulated in table no 3.
Disintegration test:
In vitro Disintegration time was determined using tablet disintegration apparatus (USP apparatus). The test was performed using disintegration apparatus with distilled water as medium. The water was heated up to 370C. The tablets were dropped in each of 6 tubes of the apparatus. Time in second for complete disintegration of tablets was measured.
In-vitro dissolution:
In vitro dissolution drug release was carried out using a digital tablet dissolution test apparatus (Veego Model No DA- 6D) in 900 ml of pH 7.4 phosphate buffer at 37�0.50C at 75 rpm using USP type II apparatus Aliquot were withdrawn at 5, 10 15, 20, 30 and 45 min and were replaced immediately with the same volume of the fresh buffer. Aliquot were diluted suitably and assayed spectrometrically (Shimadzu model 1700) at 274nm. The data is tabulated in table no 3.
Result and Discussion
Preformulation characteristics of the mucilage contribute in the formulation of the efficient fast dispersing tablets with natural disintegrants. The super disintegrants obtained from Lepidium sativum posses good physiochemical properties. Swelling index of the mucilage and Husk obtained from Lepidium sativum were found to be 27 and 25 respectively. The swelling factor is related to the disintegration of tablets rapid water uptake and rapid swelling leads to rapid disintegration of tablets.7,8 The LOD value is within prescribe limit as specified in official. The compressibility index and angle of repose value indicates that the mucilage and the husk powder have good flow characteristics with moderate compressibility. Different batches were formulated using different ratio of drug, mucilage and husk of the seeds of Lepidium sativum by direct compression technique. Other excipients such as sucrose, microcrystalline cellulose, talc and magnesium stearate were incorporated in the formulation. Coarser grade of Microcrystalline cellulose (PH102) was selected as diluent as it facilitate the flow property of the blend from the hopper.
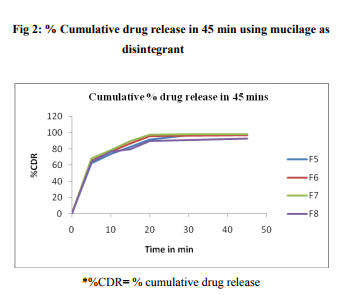
Sucrose was selected as sweetener and talc and Magnesium stearate as lubricants. In the compatibility studies no physical change was observed after 7 days. The preliminary trial was designed with 2% of disintegrant and the tablets were evaluated. Further batches were designed with increase concentration of the disintegrant. Tablets were prepared having uniform weight (200 mg) due to uniform die fill and reveled less %variation with the average. Hardness of all the formulation was kept constant (4.0-4.5 kg/cm3 ).All the formulation passes the test for friability. The drug content was found in the range of 98.50 – 102.3% (acceptable limit) The in vitro disintegration time was found to be in range of 30 to 120 sec (official NMT 3 min). It was observed that the disintegration time decreases with increase in concentration of the disintegrants. Formulation with 4% of husk and mucilage as disintegrants shows disintegration time 25 sec and 22 sec. Mucilage of seeds of Lepidium sativum has a high swelling ability and may disintegrate tablets by mainly swelling pressure. As with 5% of the husk and mucilage the Disintegration time increase that the 4%. This was due to excessive amount of the mucilage lead to excessive swelling. There was no effect of mucilage on drug release from tablets as all the formulation showed more than 90% release at 20 min at 2 to 4% of disintegrants but release rate decreases with 5% of disintegrants. This was due to higher concentration of mucilage causing excessive swelling8 . The results are showed by Fig 1 and Fig 2 for mucilage and husk respectively.
Conclusion
From the present study, it can be concluded that natural super disintegrants like Lepidium sativum mucilage and husk showed fast disintegration of tablets and can be used as Superdisintegrants in place of synthetic disintegrants.
Acknowledgement
The Authors wish to thank Panacea Biotech, Punjab for the generous gift of the sample of Diclofenac Sodium.
References:
1. Sharma S, Bharadwaj S and Gupta GD, Fast dissolving tablets of Promethezine Theoclate by Using natural Superdisintegrants, Research Journal Pharmaceutics and Technology, July-Sept 2008, 218-7.
2. Mutasem M. Rawas-Qalaji, F. Estelle R. Simons, and Simons KS. Fast-disintegrating Sublingual Tablets: Effect of Epinephrine Load on Tablet Characteristics, AAPS PharmSciTech 2006; 7(2) Article 41.
3. Setty MC, Prasad DVK, Gupta RM and SA B. Development of Fast Dispersible Aceclofenac Tablets: Effect of Functionality of Superdisintegrants. Indian Journal of Pharmaceutical Sciences Mar-Apr 2008; 70 (2); 180-6.
4. Biradar SS, Bhagwati ST, Kuppasad IJ, Fast Dissolving Drug Delivery Systems: A Brief Overview, The Internet Journal of Pharmacology, 2006 Volume 4 Number 2.
5. Patel DM, Prajapati DG and Patel NM. Seed Mucilage from Ocimum americanum Linn. as disintegrants in Tablets : Separation and Evaluation. Indian Journal of Pharmaceutical Sciences May-Jun2007; 69 (3); 431- 5.
6. Ravikumar, Shirwaikar AA, Shirwaikar A, Prabhu SL, Rajendran MK, Studies of Disintegrant properties of seed mucilage of Ocimum gratissium, Indian Journal of Pharmaceutical Science, Nov-Dec 2007, 753-6.
7. H. Omidian1, K. Park*. Swelling agents and devices in oral drug delivery, J. Drug Del. Sci. Tech., 18 (2) 83-93 2008, 83-11.
8. Zhao Na, Augsburger LL. The influence of swelling capacity of Superdisintegrants in different pH media on the dissolution of Hydrochlorothiazide from directly compressed tablets, AAPS Pharm Sci Tech, 2005; 06(01) E120-E126. DOI:10, 1208.
9. Schiermeier S and Schmidt PC. Fast dispersible ibuprofen tablets, European Journal of Pharmaceutical Sciences Volume 15, Issue 3, April 2002, Pages 295-10
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License