IJCRR - 2(9), September, 2010
Pages: 13-22
Print Article
Download XML Download PDF
CHARACTERIZATION OF CYTOTHERAPEUTIC POTENTIAL OF SUBPOPULATION VERSUS
STEMCELLS FROM BONE MARROW
Author: Dhanasekaran Marappagoundar, Indumathi Somasundaram, Sowndarya sampath, Baskaran Mayakesavan, Rajkumar Sankaran, Sudarsanam Dorairaj
Category: Healthcare
Abstract:Research on adult stem cells has been a great deal of excitement. The candidate stem
cells present in the adult tissues are the hematopoietic and mesenchymal stem cells.
However, there exist other heterogenous cell such as side population (SP) and
endothelial progenitors (EP) that has the properties of repair and regeneration. Much
work pertaining to these subpopulations is at its infancy and more research in clinical
practice is of utmost important. Thus, the objective of this work is to find out whether
subpopulations exist in bone marrow in higher percentage and play a vital role in tissue
repair and regeneration. To test this hypothesis, we characterized stem cell populations
versus subpopulations including EP cells and SP cells from Bone Marrow samples (n =
5). Surprisingly, we found that the mean of Endoglin CD105+ CD34-, CD105+ CD90 -
for EP cells was found to be 41.68% and 35.88% respectively. The mean of ABCG2+
and CD117+ SP cells was found to be 3.58% and 4.34%. These results confirm the
hypothesis and emphasize on the fact that migration at the site of injury in vivo and
recovery not only depends on the candidate stem cells but also require the heterogenous
subpopulation cells to regulate the candidate stem cells because of the wide plasticity
and common precursor called hemangioblast. Thus, we conclude that the
subpopulations of especially ABCG2 + and CD105+ found to be a potent source of
repair mechanism and hence need to be more focused on research.
Keywords: Hematopoietic Stem cells, Mesenchymal stem cells, ABCG2+ Side population cells, Endoglin, Fluorescent Activated Cell Sorter.
Full Text:
INTRODUCTION
The therapy through cell transplant has been developed based on the adult multipotent stem cell that is becoming, consequently, an important scientific subject.1 It has already been proved that most promising stem cell source in clinical practice is represented by bone marrow with more focus on candidate stem cells especially HSC and MSC.1 However, recent development in stem cell biology has demonstrated the presence of sub population cells,
especially side population (SP) cells and endothelial progenitor (EP) cells along with stem cells which is said to possess the properties of repair and regeneration.2, 3, 4 Side population cells were first identified as a subpopulation of very primitive CD 34- negative hematopoietic stem cells (HSCs) with long term hematopoietic repopulation activity, which was characterized by their capacity to efflux Hoechst 33342 and was acquired by the expression of the ATP-binding cassette transporters such as Bcrp 1 / ABCG2.3, 5 This has been identified to produce a characteristic SP profile on basis of FACS analysis, regardless of tissue origin. Isolation of SP cells by the Hodye and FACS technique has been reported in Muscular tissue6 , Liver7 , Lung8 , Skin9 , uterus10, Testis11 , Cornea12 and Bone marrow13 . Apart from the side population cells, important subpopulation cells found to possess repair mechanism and neovascularization is Endothelial Progenitor cells that circulate in adult tissues.14 According to the initial discoveries, EPC are defined as cell positive for both hematopoietic stem cell marker such as CD34+ and an endothelial marker protein VEGFR2 as this endothelial progenitor cells have consequently been considered to device from a common precursors putatively termed a hemangioblast.15 Asahara and colleagues published that purified CD 34+ hematopoietic progenitor cells from adults can differentiate ex vivo to an endothelial phenotype.16 Much work does not exist on cell surface characterization and importance of EP cells and SP cell in Bone Marrow. Moreover, focus on homogenous candidate stem cells by sorting of CD 34+ HSC by FACS17, lineage depletion by MACS18, 19 and expansion of MSC in culture20 gained importance in cell therapy.
Hence, we speculated that heterogenous subpopulation cells play a vital role in repair mechanism and faster engraftment by regulating stem cell population of Bone marrow and not homogenous stem cells like HSC and MSC alone possess cytotherapeutic potential. To test this speculated hypothesis we analyzed the percentage of subpopulation cells especially CD 105+ Endothelial cells and CD117+ABCG2+ side population from Bone marrow ( n= 5) mononuclear cells in comparison with CD34+ HSC and CD90+ MSC using FACS. The results demonstrated in this study provide evidence for our speculation. However, further research is required to address our speculations to make bone marrow mononuclear cell therapy successful.
MATERIALS AND METHODS
Reagents used: The following antibodies conjugated with corresponding flurochromes (CD34-PE; Cat No: 348057, CD117- PE-Cy7; Cat No: 339195, CD-90-PERCP-Cy5; Cat No: 555597, Cell viability dye 7-AAD; Cat No: 555816) were purchased from BD Biosciences, (http://www.bd.com/). CD105-APC; Cat No: 17-1057 and ABCG2-PE; Cat No: 12-8888 were purchased from eBioscience, (www.ebioscience.com).
Ficoll Paque Plus; Cat No: 07917 were purchased from stem cell technologies, (www.stemcell.com). Phosphate Buffer Saline (PBS); Cat No: TL1032 were purchased from Himedia.
Bone marrow collection:
Human Bone marrow samples were obtained from the iliac crest of 5 patients with spinal cord injury – paraplegia who was aged between 25 – 40 years and who had applied for a stem cell transplantation procedure after the approval of Institutional ethics committee. Formal written consent from the donors was obtained before collection. About 10 ml of BM aspirate were collected in a syringe containing heparin to prevent coagulation.
Isolation procedure:
The bone marrow sample was diluted 1:2 with 1 X Phosphate Buffer Saline (PBS) and carefully layered on to Ficoll Paque (1.077g/mL) slowly along the sides of the tube at 45� angle to isolate Bone marrow Mononuclear cells (MNCs). The MNCs were isolated by density gradient centrifugation (400g, 30 minutes, room temperature). Further, cells were washed twice with PBS (450g, 10 minutes, room temperature) to remove residual Ficoll and other contaminants. The pellet was resuspended with RBC lysis buffer solution for 10 minutes and immediately treated with 0.9% cold NaCl to stop the lysis reaction and centrifuged (300g, 5 minutes, 4º C). Cell viability was determined using the Trypan blue dye exclusion method using hemocytometry. The mononuclear cells were characterized for various hematopoietic, mesenchymal and subpopulation cells with its surface markers using flowcytometry.
Flowcytometric protocol for
characterization:
Flow cytometry was performed on a Becton, Dickinson FACS Aria (http://www.bd.com/) using a 488-nm argon-ion LASER and 632nm red LASER for excitation; fluorescence emission was collected using its corresponding detectors. 1X106 cells were stained with appropriate amount of conjugated antibodies in each of 12X75 mm falcon polystyrene FACS tube, BD Bioscience; Cat No: 352054. The quantity of each antibody conjugated with fluorochromes added to the cells in each tube were 20μl of CD34-PE, 5μl of CD90- PER CP CY5, 20μl of CD 105-APC, 5μl of CD117-PE CY7, 20μl of ABCG2-PE, 20μl of 7- AAD (BD Via probe), respectively. All tubes were incubated for 20 minutes in dark. After incubation, cells were washed in phosphate buffer saline to remove the unbound antibodies. The pellet was further resuspended to 500μl. Data analysis and acquisition was then performed using DIVA Software, Becton Dickinson. Flow cytometer instruments were set using unstained cells. Cells were gated by forward versus side scatter to eliminate debris. The number of cells staining positive for a given marker was determined by the percentage of cells present within a gate established. A minimum of 10 000 events was characterized and recorde
DISCUSSION
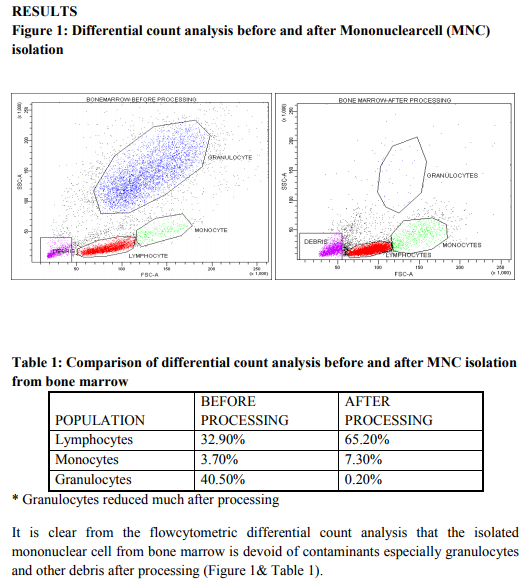
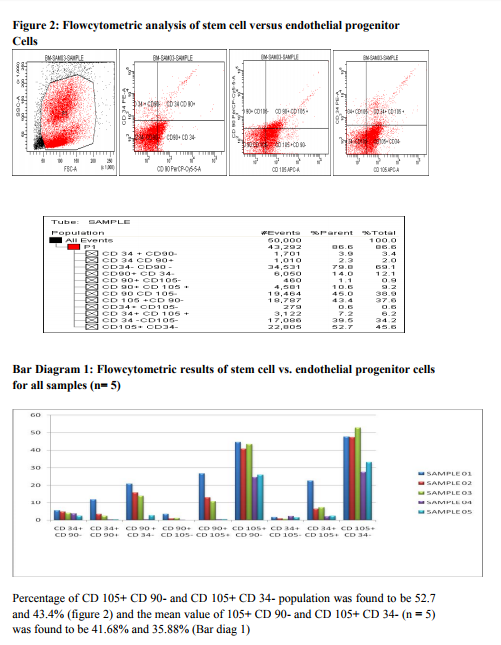
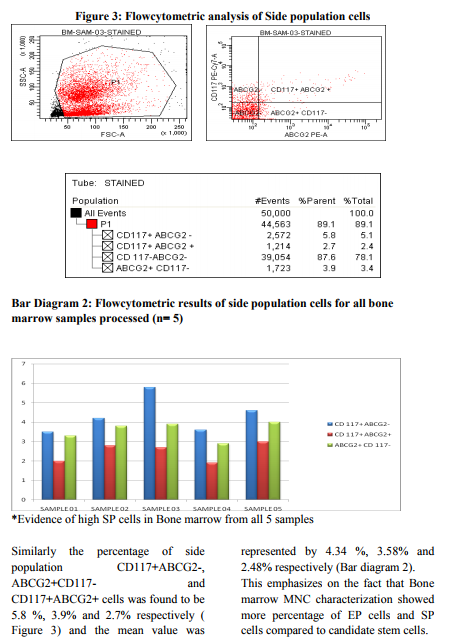
It has already been proved that Bone marrow is the promising source of stem cell therapy with presence of HSC and MSC2 . The Efficiency of the processed mononuclear cell from Bone marrow is of utmost importance before cellular characterization or cellular transplant. Thus in our study to confirm whether processed Bone marrow MNC (n=5) are devoid of contaminants and is highly efficient for further research and therapy, we performed a differential count analysis before and after MNC processing (Figure 1, Table 1). Though granulocytes live only ~10hrs in circulation, there exist many complications of granulocyte interface with MNC. It might even disrupt the stem cells from further engraftment. Hence, we emphasize on the fact that this must be an essential step for any researcher working on MNC isolation for cell transplant or research to yield better and efficient result. Recently, focus on homogenous candidate stem cells by sorting of CD 34+ HSC by FACS17, 18 , lineage depletion by MACS19 and expansion of MSC in culture20, 21 has gained importance in research for cellular transplant than mononuclear cell (MNC) therapy. However, homogenous HSC population or MSC from Bone marrow has a vast disadvantage. MSC decreases as age increases22 and phenotypic variations exist in each MSC population from various sources.23 Likewise, pure HSC population exhibit less percentage and are not plastic as well compared to heterogenous MNC.24 Moreover, by isolating or sorting pure HSC/MSC population for transplant, faster engraftment and recovery might not be fully satisfied. Existing evidence suggests that EP cells of CD34+CD133+Flk+ possess efficient repair mechanism of neovascularization in Ischemia.4, 14, 15 Much work on EP cell plasticity is not widely studied and CD 105+ Endoglin which is said to be putative EP cell population is also not much focused in research. Likewise, Presence of side population cells has been shown in many adult tissues and the SP phenotype might be represented as a common molecular regulatory feature for a wide variety of stem cells.5 However, importance of CD117+ABCG2+ SP cell research exists much only on tumor cell not in bone marrow cells.25, 26 Thus we speculated that Bone marrow, being heterogenous, not only contain HSC/MSC but also subpopulation especially EP & SP cells which serves to be an effective repair mechanism. To confirm our speculated hypothesis, in our experiment, we analyzed the percentage of subpopulation cells especially CD 105+ EP cells and CD117+ABCG2+ SP cells from Bone marrow ( n= 5) mononuclear cells in comparison with CD34+ HSC and CD90+ MSC using FACS. The results demonstrated in this study provide evidence for our speculation. We found that Bone marrow MNC contain more percentage of CD 105+ EP cells compared to CD105+ cells obtained from other research works of Takayuki Asahara et al16. We also found that CD105+ EP cell percentage is found to be much higher compared to CD34+CD133+ EP cells obtained from Carmen Urbich et al, 2006 and Mihali Hristov et al, 20034, 14. Similarly, several researchers have demonstrated that the percentage of SP cells from bone marrow ranges between 0.01- 3.0 %.3,5 In contrast to the existing data, the percentage of ABCG2+CD117+ SP cells in our results from all 5 samples was found to be much higher (Figure 3& Bar diagram 2). Moreover, the percentage of CD105+ and CD117+ABCG2+ from bone marrow is found to be higher than the percentage obtained from stromal vascular fraction of subcutaneous and omentum fat tissue from our previous research work.27 This confirms that the Subpopulation cells are exhibited in higher percentage in bone marrow compared to adipose tissue. Finally it is evident from our research work that the migration at the site of injury and recovery in vivo not only depends on the candidate stem cells ( HSC and MSC) but also require the heterogenous subpopulation (EP and SP) cells to regulate the candidate stem cells. Thus, we emphasize that Bone marrow mononuclear cells are the best source of clinical transplants unlike homogenous HSC and MSC population because of the vast heterogeneity and plasticity. However, further research on these subpopulation cells will bring this work closer to clinical applications.
References:
1. Ana maria cristina serban, Gabriela Tanasie, Daciana Nistor. ?Stem cell types in Bone marrow?. TMJ 2008; 58: 3-4.
2. Challen GA, Little MH. Aside order of stem cells: The SP Phenotype. Stem cells 2006; 24: 3-12
3. Margaret A. Goodell, Shannon McKinney-freeman and Fernando D. Camargo Isolation and characterization of side population cells. Mol bio 1996; 290.
4. Carmen Urbich and Stefanie Dimmeler. Endothelial progenitor cells: Characterization and role in vascular biology. Circ.Res 2006; 95: 343-353.
5. Guo Y, Follo M, Geiger K, Lubbert M, Engelhard M. Side population cells from different precursor compartments. Stem cell Res 2003; 12: 71-82.
6. Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001; 107:1395–1402.
7. Shimano K, Satake M, Okaya A, Kitanaka J, Kitanaka N, et al. Hepatic oval cells have the side population phenotype defined by expression of ATP-binding cassette transporter ABCG2/BCRP1. Am J Pathol 2003; 163:3–9
8. Majka SM, Beutz MA, Hagen M, Izzo AA, Voelkel N, Helm KM. Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells 2005; 23:1073–1081.
9. Yano S, Ito Y, Fujimoto M, Hamazaki TS, Tamaki K, Okochi H. Characterization and localization of side population cells in mouse skin. Stem Cells 2005; 23:834–41.
10. Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci USA 2007; 104:18700–5.
11. Lassalle B, Bastos H, Louis JP, Riou L, Testart J, Dutrillaux B, Fouchet P, Allemand I. 'Side Population' cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development 2004; 131:479–87.
12. Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005; 23:1266–1275.
13. Jihyun Yoon, Seung- Cheol Choi, Chi-yeon park, Ji-Hyun choi, Yangin Kim, Wan-Joo Shim and Do-sun Lim. Bone marrow derived side population cells are capable of functional cardiomyogenic differentiation. Mol. Cells 2008; 25,2:216-223.
14. Mihail Hristov, Wolfgang Erl and Peter C. Weber), Endothelial progenitor cells: Mobilization, Differentiation, and Homing. Arterioscler Thromb Vasc Biol 2006; 23:1185-1189.
15. Taka yuki Asahara, Haruchika Masuda, Tomono Takahashi, Christoph Kalka, Christopher Pastore, Marcy Silver, Marianne Kearne, Meredith Magner, Jeffrey M. Isner. Bone marrow origin of Endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological Neovascularization. Circ. Res 1999; 85: 221-228.
16. Asahara T, Murohara T, Sullivan A, Silver M, Van der zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275:964-967.
17. Loken MR. Immunoflourescence techniques in flowcytometry and sorting. Weiley 1990; 341-353.
18. Dhahlke MH, Lauto OS, Jager MD, et al.. In vivo depletion of hematopoietic stem cells in the rat by an anti- CD45 (RTT) antibody. Blood 2002; 35: 66-72
19. Forraz N, Pettengell R, McGuckin CP. Characterization of a lineage negative stem progenitor cell population optimized for ex vivo expansion and enriched for LTCIC. Stem cells 2004; 22(1): 100 - 8.
20. Granthos , Zannettino AC. A method to isolate and purify bone marrow stromal stem cells. Methods Mol Bio. 2008;449:45-57
21. Le Blanc K. Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy 2006; 8:559–561.
22. Mueller SM, Glowacki J. Agerelated decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional 22 International Journal of Current Research and Review www.ijcrr.com Vol. 02 issue 9 Sep 2010 collagen sponges. J Cell Biochem 2001; 82:583–590.
23. Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005; 33:1402–1416.
24. Laurent roybon, zhi ma, fredrik asztely, et al. Failure of Transdifferentiation of Adult Hematopoietic Stem Cells into Neurons. STEMCELLS 2006; 24:1594–1604
25. C.Hirschmann-jax, Foster. A.E, Wulf.G.G, Nuchtern.J.G, Jax.T.W, Gobel.U, Goodell.M.A., Brenner.M.K. A distinct side population of cells with high drug efflux capacity in human tumor cells. 2004.
26. Colleen Wu, Qingxia Wei, Velani Utemo et al. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer research 2007; 67: 8216.
27. Dhanasekaran Marappagoundar, Indumathi Somasundaram et al. Characterization of Hematopoietic stem cells, mesenchymal stem cells and side population cells from adipose tissue. International Journal of Biology 2010; 2(1): 71-78.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License