IJCRR - 3(4), April, 2011
Pages: 04-12
Print Article
Download XML Download PDF
TASTE MASKING OF PIROXICAM BY SPRAYDRYING TECHNIQUE
Author: Ashwini G Kini, Mudit Dixit, P.K Kulkarni, Venkatesh.A, Akash Johri
Category: Healthcare
Abstract:Piroxicam is a Non-steroidal anti inflammatory, analgesic and anti-pyretic drug. This is widely
used in Muscular-skeletal disorder like osteoarthritis. Piroxicam has bitter taste, half life of 30
hrs and poor water solubility. So the present work was focused on masking the bitter taste of
piroxicam. In the present research was to develop the taste-masked microspheres of bitter drug
piroxicam by spray-drying technique. The bitter taste threshold value of piroxicam was determined. Three different polymers viz. low molecular Chitosan, high molecular Chitosan and Pluronic F127 were used for piroxicam microsphere formation, and the effect of different polymers and drug\?polymer ratios on the taste masking and release properties of microspheres was investigated. The microspheres were characterized by Fourier transform infrared spectroscopy, Differential scanning colorimetric, scanning electron microscopy, Drug loading,
in vitro bitter taste evaluation, and drug-release properties. The taste masking was found absent in low molecular chitosan microspheres at 1:1 drug\?polymer ratios. The high molecular chitosan microspheres showed taste masking at 1:2 drug\?polymer ratio, whereas with Pluronic F127 microspheres the taste masking was not achieved at all the drug\? polymer ratio. The drug release was about 53.34% for low molecular chitosan microspheres and 39.57% for high molecular Chitosan microspheres in 15 min. hence spray dried microspheres can be effective
technique for taste masking of bitter drugs without affecting the drug property.
Keywords: microspheres; spray drying; piroxicam, taste masking.
Full Text:
INTRODUCTION
The biological definition of taste (Gustation) is a chemical reaction derived from sensory responses from the four main taste perceptions: salt, sour, bitter, and sweet. Taste sensation is the result of signal transduction from the receptor organs for taste, commonly known as taste buds. The taste buds contain very sensitive nerve endings which produce and transmit electrical impulses to the brain. The perception of taste only occurs when the substances are dissolved in mouth. The drug substance first gets solubilized in saliva and then they interact with the taste buds and perception of taste occurs [1,2]. Taking medicine orally is convenient and economical. It also requires cooperation from the patient. Unfortunately, many drugs have unpleasant taste primarily bitter. This has led to dilemma for modern pharmaceutical science as undesirable taste can hinder the acceptance and usefulness of many beneficial, safe, and efficacious drugs. Thus, elimination or reduction of bitterness is an important mainstay of product evaluation in oral pharmaceutical formulation. Numerous approaches have been reported for masking the bitter taste of the drugs such as [1] use of flavors and sweeteners, [2] use of polymeric carriers, [3] drug resin complexes, [4-7] formation of inclusion complexes, etc. Taste masking by polymeric coating involves formation of a physical barrier between drug particle and the taste bud, thus, minimizing the interaction. Polymeric coating retards the release of the drug in oral cavity, thus, prevents the interaction of drug with taste buds. Various hydrophilic and hydrophobic polymers such as hydroxylpropyl methyl cellulose, ethyl cellulose, polymethacrylates, microcrystalline cellulose, etc. are reported for taste masking. The method used for taste masking with polymers includes wet granulation, fluidized bed coating, microencapsulation, etc. [7-14]. Piroxicam is selected as a suitable candidate for taste masking due to its bitter taste. Piroxicam is a Non-steroidal anti inflammatory, analgesic and anti-pyretic drug which is widely used in Muscular-skeletal disorder like osteoarthritis. Piroxicam has bad taste, half life of 30 hrs and poor water solubility. The present investigation is aimed at use of polymers as a taste-masking agent like low molecular Chitosan, high molecular Chitosan and Pluronic F127 are used to prepare microspheres. Low molecular weight (150,000) chitosan is 75-85 percent deacetylated and has a viscosity of 20-200 cps) / High molecular weight (600,000) chitosan is a coarsely ground polymer prepared from crab or shrimp shells with a viscosity of 800-2000 cps) molecular weight) polycationic polysaccharide derived from naturally occurring chitin and is insoluble above pH 6 and it dissolve readily in diluted solution of several organic acids including formic, acetic, tartaric, citric and lactic acid (Rowe et al. 2004). Pluronic F-127 is a nonionic, surfactant polyol (molecular weight approximately 12,500 daltons) that has been found to facilitate the solubilization of water-insoluble dyes and other materials in physiological media. Spray drying was used for the preparation of the microspheres. Spray drying is widely used in pharmaceutical processing as it requires only a one-step process and can be easily controlled and scaled up.
METHODS AND MATERIALS
Piroxicam (PIRX), low molecular weight chitosan (L.M.C), high molecule weight chitosan (H.M.C) and pluronic F 127 (PLU-F127) were gifted by IPCA lab, Mumbai, (India). The other chemicals and reagents used were of AR grade.
Preparation of Microspheres
The microspheres were prepared by spraydrying technique. The spray drying was performed by Mini Spray Dryer LSD -48; (Jay instrument and systems Pvt. Ltd. Mumbai). The different drug–polymer ratios used for various microsphere formulations were prepared described in Table 1. The polymer solution was prepared by adding given quantity of polymer to the solvent. For low and high molecular weight chitosan 1% glacial acetic acid and water were used as solvent mixture, whereas for pluronic F127, Dichloromethane was used as solvent (15). The given quantity of piroxicam was added to the polymer solution and the resulting mixture was spray-dried. The spray drying parameters are described in Table 2
Evaluation of taste masked
Microspheres Percentage Yield of spray dried microspheres
The yield of microspheres was determined by the formula,
Drug loading of spray dried microspheres
The drug loading was determined by UVVisible spectrophotometer. The microspheres were stirred with 100 ml 0.1 N HCl for 2 h. The drug concentration was determined at 334 nm after suitable dilution. The readings were taken in triplicate.
Determination of bitter taste recognition threshold value of piroxicam
The bitter taste threshold value of piroxicam was determined based on the bitter taste recognized by five volunteers (three males and two females) in the age group of 18– 25 years. Aqueous solutions of piroxicam with different concentrations (2, 4, 8, 13, and 18μg/ml) were prepared. One milliliter of solution was placed on the center of the tongue of volunteer for 30 s. The solution was spat out after 30 s, and the mouth was thoroughly rinsed with distilled water. The same procedure was repeated for all solutions and volunteers. A gap of 1 hr was maintained in between tasting two different solutions. The same procedure was repeated for piroxicam solutions with concentrations 4.5, 5.5, 6.5, and 7.5μg/ml. The threshold value was selected on the basis of the lowest concentration that had a bitter taste [4, 15,16, 17].
In vitro bitter taste evaluation of microspheres
Microspheres (equivalent to 8 mg of piroxicam) were placed in a volumetric flask with 25 ml of phosphate buffer pH 7.4 and stirred for 5 min. The mixture was filtered, and the filtrate was analyzed for piroxicam concentration at 334 nm by UVVisible spectrophotometer method (UV 1601 A Shimadzu, Japan) and that was compared with the threshold value.
Fourier transforms infrared spectroscopy (FTIR)
The FTIR spectral measurements were taken at ambient temperature using a Shimadzu, Model 8033 (USA). Samples were dispersed in KBr powder and the pellets were made by applying 5 ton pressure. FTIR spectra were obtained by powder diffuse reflectance on FTIR spectrophotometer.
Scanning electron microscopy (SEM):
Scanning electron microscopic (Joel- LV- 5600, USA, with magnification of 250x) photographs were obtained to identify and confirm spherical nature and Surface topography of the microspheres.
Dissolution studies of microspheres:
The dissolution of piroxicam pure sample, spray dried microspheres formulations were determined by using USP dissolution apparatus XXIV-Type II (Electro Lab, Mumbai). Dissolution medium was 900 ml 7.4 Phosphate buffer. The amount of dissolved drug was determined using UV spectrophotometric method (UV 1601 A Shimadzu, Japan) at 334 nm. The readings were taken in triplicate.
RESULTS
The glass transition temperature (Tg) is the second-order phase change temperature at which a solid glass is transformed to a liquid-like rubber. As the temperature increases above, Tg various changes, such as increase of free volume, decrease of viscosity, increase of specific heat, an increase of thermal expansion, are noticed. During spray drying, if the drying temperature exceeds the Tg of the polymer, the powder becomes soft or sticky while still warm. This cause The Sticking of powder to the side walls of drying chamber.
Drug Loading and % yield
Table 4, summarizes the results of drug loading and production % yield.
Determination of Bitter Taste Recognition
All the Five volunteers could not recognize the bitter taste of Piroxicam at 4.5 and 6.5μp/ml. four out of five volunteers can percept the bitter taste at 9.5pg/ml, whereas all the seven volunteers reported that the solutions of 14.5 and 19.5pg/ml were bitter.
In Vitro Bitter Taste Evaluation of Microspheres
The drug release in pH 7.4 phosphate buffer was studied to evaluate taste masking. The drug release from Low molecular weight chitosan microspheres (drug-polymer ratio 1:1) and High molecular weight Chitosan microspheres (drug–polymer ratio 1:2) was less than the threshold bitterness value, i.e., 7.5μg/ml. The drug release for Pluronic F-127 microspheres was above the threshold value for all the drug– polymer ratios studied.
Infrared Spectroscopy studies
The FTIR spectrum of drug, polymer, drug and different drug: polymer ratio microspheres (PIRX: L.M.C, PIRX: H.M.C, PIRX: PLU-F-127) are showed in Fig. 1.
Scanning Electron Microscopy
The SEM micrographs of pure piroxicam, low molecular weight chitosan Microspheres, high molecular weight Chitosan Microspheres are depicted in Fig. 2.
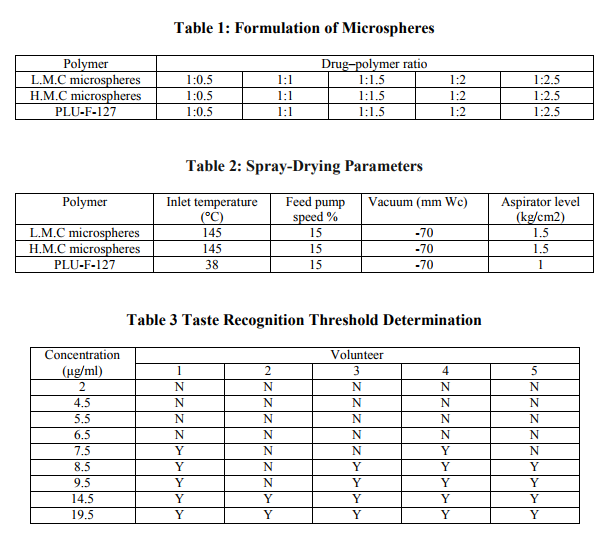
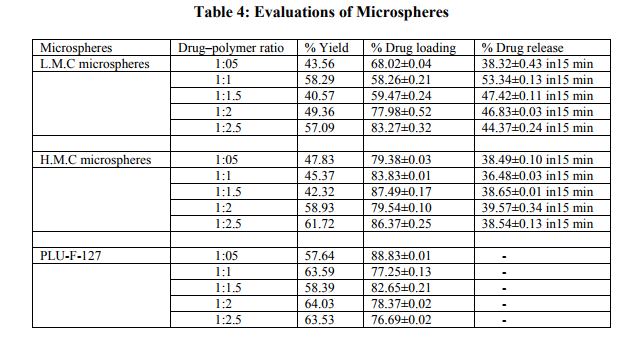
In-vitro Drug Release
The drug release results are depicted in Table 4. The Low molecular weight chitosan and high molecular weight Chitosan microspheres passed the bitterness evaluation test; therefore, they were selected for drug-release study (Fig. 3).
DISCUSSIONS
The Tg of Pluronic F127 as provided by the manufacturer is 42°C so dichloromethane was selected as solvent with boiling point 36°C, i.e., lower than the Tg of pluronic F 127, whereas Tg of low and high molecular weight of Chitosan is between 152- 203°C,(14). Therefore, water and 1% glacial acetic acid were used as solvent for Low and High molecular weight Chitosan microspheres (Sohi, H. et al. 2004). The spray dried microspheres formulations collected and were free-flowing and white in color. The percentage yield of spray dried microspheres showed in table 4. Drug loading for the spray dried microspheres formulation was showed in Table 4. The threshold bitterness value lies in between 4.5 and 9.5μg/ml. Therefore, the piroxicam solutions of 5.5, 6.5, 7.5, and 8.5 μg/ml concentrations were prepared, and the same procedure was repeated. From Table 3, the bitter taste threshold value of piroxicam is 7.5μg/ml (Khan, S. et al. 2007). The microspheres were prepared with different drug to polymer ratios. The Low molecular weight exhibited excellent taste masking at drug–polymer ratio 1:1. Taste masking was also achieved at drug– polymer ratio 1:2 and 1:2.5. All the other ratios studied did not show taste masking as the drug release at pH 7.4 phosphate buffer was above the threshold bitterness value. This may be because of incomplete film formation by the Low molecular weight which fails to control the release of piroxicam at salivary pH. Chitosan is insoluble in alkali solutions at pH above 6. High molecular weight Chitosan exhibited taste masking at drug–polymer ratio 1:2 and 1:1.5 but taste masking was not achieved for other ratios (Khan, S. et al. 2007). The PIRX exhibited characteristic peaks at showed characteristic peaks in all the spectra‘s like –NH and –OH stretching which ties at 1385 cm-1 , 1635 or 1625 cm-1 (N-H-CO3 stretching vibration), 1525 cm-1 (secondary -NH2 stretching), 1440 cm-1 (CH3 AND Ar-c=c stretching), 1355 cm-1 (sym. –CH3) and 1155 and 1070 cm-1 or 1050-1070 cm-1 (-SO2-N-) 770 and 740 or 740 cm-1 ( Ortho-disubstituted phenyl). The Low molecular weight chitosan microspheres exhibited both the characteristic peaks of PIRX at 1385 and 1635cm−1 . High molecular weight Chitosan microspheres depicted no shift in both the characteristic peaks of PIRX. The results of IR reveal that there was no chemical interaction between drug and polymers. The microspheres prepared by spray drying were spherical in shape with small diameter in the range 4–11μm. The SEM images confirmed the uniformity and fine nature of the microspheres which contributed for rapid drug release from the microspheres. The low molecular weight chitosan microspheres (1:1 drug–polymer ratios) showed 53.34% release in 15 min, whereas high molecular weight Chitosan microspheres (1:2 drug–polymer ratio) depicted 39.57% release in 15 min. This could be due the spherical and uniform size of microspheres or increasing the wetability of microspheres. CONCLUSION Spray-dried microspheres of Low molecular weight chitosan and High molecular weight Chitosan depicted excellent taste-masking ability. Low molecular weight chitosan did not affect the drug release whereas high molecular weight Chitosan exhibited slight delay in drug release as compared to Low molecular weight chitosan, but the slight delay can be outweighed by the virtue benefit achieved of taste masking and better patient compliance.
ACKNOWLEDGEMENTS
The authors are thankful to IPCA labs, Mumbai, India for the gift sample of piroxicam, and Principal, J.S.S. College of Pharmacy, Mysore for providing facilities to carry out this work.


References:
1. Jacob, T. (2006). Taste—A brief tutorial by Tim Jacob, http://www.cf.ac. uk/biosi/staff/Jacob /teaching /sensory/taste.html, 23 Aug.
2. Romanov, R A and Kolesnikov, S. S. (2006). Electrophysiologically identified subpopulations of taste bud cells. Neurosci. Lett. 395:249–254.
3. Sohi, H. Khar, R. K. and Sultana, Y. (2004) Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev. Ind. Pharm. 30:429–448.
4. Gao, Y. Cui, F. Guan, Y. Yang, L. Wang, Y and Zhang L. (2006),Preparation of roxithromycinpolymeric microspheres by the emulsion solvent diffusion method for taste masking. Int. J. Pharm. 318:62-69.
5. Shen, R. W. (1996), Taste masking of ibuprofen by fluid bed coating. US Patent 5, 55,152, September 3.
6. Lorenzo L. M., Kuna, M and Vila J. J. (1997), Development of microencapsulated form of Cefuroxime axetil using pH sensitive acrylic polyme
7. Khan, S. Katariya, S. P. Nakhat, S and Yeole, P. (2007), Taste masking of ondansetron hydrochloride by polymer carrier system and formulation of rapid disintegrating tablets. AAPS PharmsciTech. 8(2):E1–E7.
8. Manek, S. P and Kamat, V. S. (1981), Evaluation of Indion CRP-244 and CRP-254 as sustained release and taste masking agents. Indian J. Pharm. Sci. 43(11–12):209–212.
9. Borodkin, S. (1971), Sundberg. Polycarboxylic acid ionexchange resin adsorbates for taste coverage in chewable tablets. J. Pharm. Sci. 60:1523–1527.
10. Sriwongjanya, M and Bodmeier, R. (1997), Entrapment of drug loaded ionexchange particles within polymer microparticles. Int. J. Pharm. 158:29– 38.
11. Szejtli, J and Szente, L. (2005), Elimination of bitter, distinguishing taste of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 61:115-125.
12. Cox, E. H. Beal, S. L. Follet, C. V. Fuseau, E. Kenkare, S and Sheiner L. W. (1999), A population pharmacokinetic-pharmacodynamic analysis of repeated measures time-toevent pharmacodynamic Taste Masking 1163 responses: the antiemetic effect of ondansetron. J. Pharmacoknet. Biopharm. 27(6):635– 644.
13. Johnson, B. A. Cowen, P. J and Rue, j. (1993), Ondansetron and alcohol pharmacokinetis. Psychopharmacology. 112:145.
14. Rowe, R. C. Owen S. C and Sheskey, P. J. (2004), Handbook of Pharmaceutical excipients, 4th ed., KM Varghese Company, Mumbai.
15. Goula, A. M. Achilias, D. S. Adamopoulos, K. G and Karapantsios, M. D. (2008), Water sorption isotherms and glass transition temperature of spray dried tomato pulp. J. Food Sci. 85:78–83.
16. Albertini, B. Cavallari. Magarotto, L. Rodriguez, L. Passerini, C. N and Voinovich, d. (2004), Characterization and taste masking evaluation of acetaminophen granules: comparison between different preparation methods in a high shear mixer. Eur. J. Pharm. Sci. 21:295–303.
17. Chang, W. Chung, J. W. Chung, S. Kim, Y. and Kho, H. (2006), The relationship between phenylthiocarbamide (PTC) and 6-npropylthiouracil (PROP) taster status and taste thresholds for sucrose and quinine. Arch. Oral Biol. 51:427–432.
18. The United Stated Pharmacopeia XXIII/National Formulary XVIII. US Pharmacopoeial convention, Rockville, MD, 1980:2800.
19. Bora. D. Borude P and Bhise K. (2008), Taste Masking by Spray-Drying Technique, AAPS PharmsciTech, 8: 1159- 1164.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License