IJCRR - 3(9), September, 2011
Pages: 167-179
Print Article
Download XML Download PDF
BACOPA MONNIERI L. (HIGHLY ENDANGERED SPECIES): AN IMPROVED MICROPROPAGATION PROTOCOL AND RAPD ANALYSIS OF GENETIC VARIABILITY OF NATURAL AND MICROPROPAGATED
PLANTS FOR GERMPLASM PRESERVATION.
Author: Renu Sharma, Moinuddin Khan
Category: General Sciences
Abstract:Bacopa monnieri has been used in ayurvedic system of medicine for centuries. It is great neurotonic,
immuno-modulator, adaptogen, tranquilizing, memory and learning enhancing, cerebral activator, antiulcer,
anti-asthmatic ayurvedic herb. Thus the micro-propagation of this plant is very necessary to
maintain the commercial demand and to preserve the germplasm of this endangered plant. The objective
of this study is to determine the easy and cost effective micro-propagation protocol. Direct shoot
regeneration was obtained on MS basal medium supplemented with different PGRs. Highest no. of
micro-shoots (128) produced on BAP (0.1 mg/l) using leaf explant which had been previously inoculated
on BAP (2.0 mg/l) i.e. shoot initiation medium. However nodal segment gave 88.2 shoots on BAP (0.5
mg/l), longer shoots (13.0cm) obtained on BAP (1.0 mg/l) + Kn (0.5 mg/l) using leaf explants. Best
response in in vitro rooting (13.1) achieved on MS ½ + IBA (1.5 mg/l) and longest roots (6.4) occurred on
MS ½ + NAA (1.0 mg/l). Rooted plantlets after acclimatization were transferred to green-house
conditions they show 100% survival rates. A key step in the analysis of plant DNA restriction fragments
is the purification of sufficient quantity of good quality DNA from fresh and healthy plant tissues, this
technique is ideal for isolation of DNA from Bacopa plants and the isolated DNA was used for RAPD
analysis. We used six primers in this analysis, all the profiles of DNA for all the smaples, were gave
similar results. This indicates that the abscence of intraspecific polymorphism among the samples.
Keywords: Bacopa monnieri, ayurvedic herb. , micro-propagation, RAPD analysis
Full Text:
INTRODUCTION
Bacopa monnieri (Coastal Waterhyssop, Brahmi, water hyssop) belongs to the Scrophulariaceae . This is a perennial , creeping herb whose habitat includes wetlands and muddy shores. It commonly grows in marshy areas throughout India, Nepal, Sri lanka, China, Taiwan and Vietnam, and is also found in Florida and other southern states of the USA where it can be grown in damp conditions by the pond or bog garden. The leaves of this plant are succulent and relatively thick. Leaves are oblanceolate and are arranged oppositely on the stem. The flowers are small and white, with four or five petals. Its ability to grow in water makes it a popular aquarium plant. It can even grow in slightly brackish conditions this plant has a number of uses in Ayurveda. It is a traditional treatment for epilepsy and asthma, Rajani et al., (2004). It has been used for centuries in folklore and traditional system of medicine as a memory enhancer, anti-inflammatory, analgesic, antipyretic, sedative and anti-epileptic agent (Stough et al.,2001). It has antioxidant properties, reducing oxidation of fats in the bloodstream. However, anti-epilepsy properties seem to be in very high toxic and near lethal doses, so it's only used at much lower non-toxic dosage as a (cognitive) additive to regular epilepsy medication. Studies in humans show that an extract of the plant has antianxiety effects Rajani et al., (2004). Bhrami extract is known to also have anti-cancer and antioxidant properties (Elangovan et al., 1995; Tripathi et al., 1996). The saponins present in the plant, namely Bacosides A and B, have been indicated for memory-enhancing properties (Sing et al., 1988). It is listed as a nootropic, a drug that enhances cognitive ability. In India, this plant has also been used traditionally to consecrate newborn babies in the belief that it will open the gateway of intelligence. Laboratory studies on rats indicate that extracts of the plant improve memory capacity and motor learning ability Rrajani et al.,(2004). Recent studies suggest bacopa may improve intellectual activity. Stough et al.,(2001), Roodenrvs et al., ( 2002), Stough et al., (2008), Dhanaskheran et al., (2007) .This mechanism of action may explain the effect of Bacopa monnieri extract in reducing beta-amyloid deposits in mice with Alzheimer's disease. The multiple uses of this important medicinal plant have led to its indiscriminate collecting and use. It is estimated that about 100000 tonnes of brahmi material is collected from the wild every year for commercial use in India (Ahmad 1993; Mathur 1999). Due to its great medicinal value there is an urgent need to develop alternative, rapid, simple and efficient protocol for the in vitro microprogation of B. monnieri. Through micropropagation of Brahmi has been reported by various authors (Ali et al.,1999; Mathur and Kumar 1998; Srivastava and Rajani 1999; Tejavathi and Shailaja 1999; Tiwari et al., 1998, 2000; Misra et al., 2003; George et al.,. 2004).
MATERIALS AND METHODS
1. Collection of explants and surface sterilization:- Bacopa monnieri plants were collected from botanical garden of Quila at Aligarh Muslim University, Aligarh. Nodal segments, intermodal segments, shoot tips, leaf were selected as explants for direct regeneration, The explants were washed thoroughly under running tap water for 30 minutes followed by 4-5 drops of mild liquid detergent Lavolene (Qualigens Fine Chemicals , Bombay India) for 10 minutes . After that explants were soaked in an aqueous solution containing 0.2 % (w/v) Bavistin (BASF , Mumbai India Limited) for 10 minutes. The explants were then washed repeatedly with distilled water and finally treated with HgCl2 0.1 % (w/v) (Hi Media , Mumbai India) for 5 minutes in a laminar air flow chamber and washed at least five times with sterile distilled water to remove any traces of HgCl2. Then washed in 70 % alcohol for 1 minute after that rinsed properly with sterile double distilled water for at least two times . 2. Culture media:- Murashige and skoog (1962) medium were used as basal medium supplemented with 3 % (w/v) sucrose (Hi Media, Mumbai India). Ph of this medium was adjusted to 5.8 by using 1 N NaOH/ HCl before adding 0.8 % (w/v) agar (Hi Media, Mumbai India). Based on the experiment , different concentration of different plant growth regulators were added separately or in combination. Then this medium was autoclaved at 1210 C for 20 minutes.
3. Initiation of cultures :- Sterilized explants were transferred to aseptically to sterilized glass plate in the laminar air flow chamber , cut the top portion of explant to remove dead portion after surface sterilization. The forcep and the blade were earlier rinsed in the 70 % alcohol and flamed and cooled , were used . Each explant placed in an erect position on the media ant these jars were sealed with kiln film. After each inoculation the forcep and blade wre rinsed in 70 % alcohol to reduce the contamination . These glass bottles or jars or culture tubes were incubated in growth chamber / room at 25± 2 0 C with a photo- period of 16 hrs and 8 hrs dark period under fluorescent light (2500 lux ) and relative humidity of 50-60 % . Each experiment had three replicates with ten cultures.
4. Multiple shoot regeneration:- From various inoculated explants multiple shoots were produced after 10-15 days . These microshoots needs sub-culturing after two / three weeks for proper growth.
5. Root induction:- Micro shoots were transferred to a rooting medium . Under laminar air flow chamber micro shoots up to 2 cm long were cut with the help of sterile forcep and scalpel and placed on rooting medium and finally incubated in growth room.
6. Acclimatization:- Rooted plants were removed from culture tube and washed under tap water to remove agar , transferred to plastic cups containing sterile soil rite +soil mixture . The plantlets were covered with polythene bags to maintain high humidity 90± 5 % and irrigated after every 2 days with half-strength MS salt solution (without vitamins) for 2 weeks. Polythene bags were removed after 2 weeks. When new leaves appeared, the plants were transferred to earthen pots containing garden soil and vermin-compost (1:1), the plants were transferred to green-house conditions for two weeks and finally brought in natural sun light.
RAPD Analysis:- Random amplified polymorphic DNA analysis was carried out to check the polymorphism between mother plants and in vtro generated plants. In this protocol firsty DNA was isolated from different in vitro generated plants whose medium supplemented with different types of plant hormones alone or in combinations , were screened for 6 primers. These primers were used as GBP-01, GBP-02, GBP-03, GBP-04, GBP-05 and GBP-06 respectively. Each RAPD reaction mixture comprised of 25 ng of genomic DNA, 20 pMoles of the appropriate RAPD primer; 200 pM of dATP, dCTP, dGTP and dTTP; and 1.0 U of Taq DNA polymerase and 1XPCR buffer (provided in the kit). Reaction products were analyzed by electrophoresis through 1.5% (wt/vol) agarose (Promega Corporation, Madison, WI 53711 USA) gel slabs (10 cm by 16 cm by 6 mm) with 0.5 x Trisborate- EDTA as the resolving buffer. Gels were stained with ethidium bromide, placed over a source of UV light (260 nm), and then photographed using a BIORAD Gel documentation system. The molecular sizes of DNA fragments were determined using GeneRuler 1kb Ladder (Fermentas International Inc, Canada) DNA size standard. Statistical analysis:-All the experiments were conducted with a minimum of ten replicates per treatment and repeated two times.
RESULT
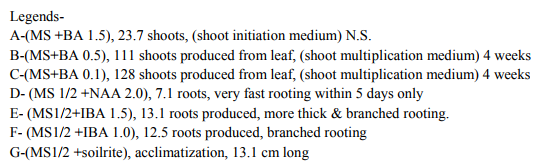
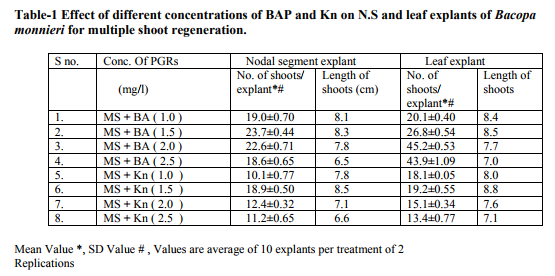
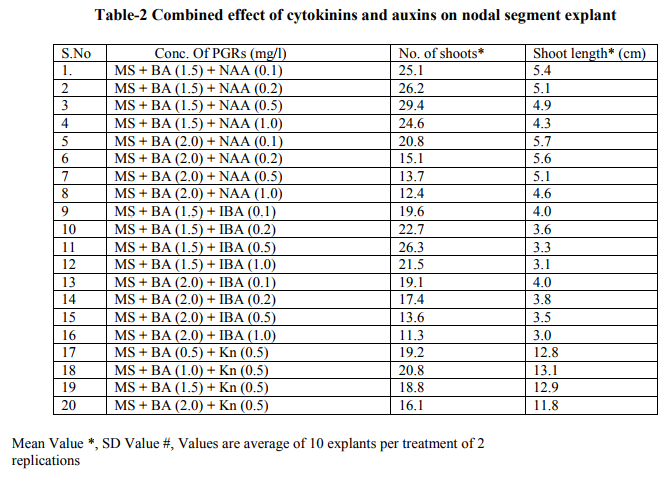
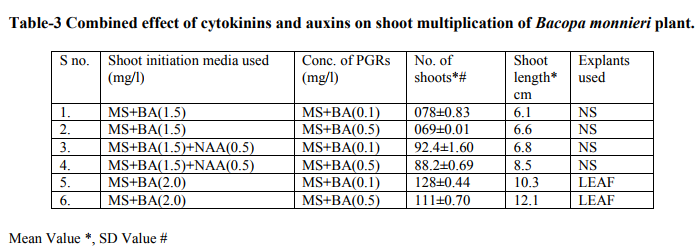
Bacopa monnieri plant were efficiently regenerated from different explants eg. Shoot tips, nodal segments, inter nodal segments, leaf. All the explants highly capable of develop micro-shoots directly on MS basal media containing various plant growth regulators alone or in different combinations. Explants were extremely sensitive to Hgcl2 therefore we used the 0.1 % (w/v) for 2 minutes only not for 5 minutes because explants showed browning with contamination .Before using Hgcl2 solution we used a fungicide bavistin 0.2 % (w/v) for 10 minutes for the prevention of fungi and bacteria. Shoot induction and multiplication:- In control medium explants did not show any new bud formation but it remained healthy and fresh. We used BAP and Kn (alone or in the combination). The explants respond to all the treatments but the no. of shoots and shoot length varied. When we used MS + BAP (1.5 mg/l), the highest no. of shoots i.e. (23.7) occurred within three weeks (Table 1.) figure- A. but MS +BAP (2.0 mg/l) showed slightly less results i.e. (22.6) less no. of multiple shoots. In case of Kinetin treatment, MS +Kn (1.5 mg/l) gave again less no. of shoots i.e. (18.9). Therefore in our study BAP combinations were superior over Kn combinations (Table-1). To examine the combined effect of cytokinins and auxins on explants, MS +BAP (1.5 mg/l)+ NAA(0.5 mg/l) gave best response i.e. 29.4 shoots whereas IBA added to this treatment i.e. MS+BAP (1.5 mg/l)+IBA(0.5 mg/l) gave less no. of shoots i.e. 26.3. Therefore NAA was more superior than the IBA in combination treatment on nodal segment and leaf explants (Table-2). When two cytokinins i.e. BAP and Kn added to MS basal medium, MS+BAP (1.0 mg/l)+ Kn (0.5 mg/l) average no of shoots (20.8) were developed but when we used the higher concentration of both cytokinins, less no. of shoots were developed (Table-2). We observed it this study that lower concentration of PGRs gave better response than higher concentration. Leaf explants gave best response on MS+ BAP (2.0 mg/l), highest no. of shoots were developed i.e. 45.2 shoots (Table-1). Firstly we sub-cultured the new shoots to the same treatment, approx same results were observed but when we sub-cultured to lower concentration of BAP i.e. MS+BAP(0.5 mg/l) gave best response .When we sub-cultured micro-shoots from MS+BAP(1.5 mg/l) to MS+BAP(0.1 mg/l) 78 shoots were produced within 3 weeks forming a cluster. However, micro-shoots from cytokinins and auxins combination i.e. MS+BAP (1.5 mg/l) +NAA (0.5 mg/l), sub-cultured on MS+BAP (0.1 mg/l), 92.4 new shoots were produced within 3 weeks in case of nodal segment explants. The explant which gave the most response and producing highest no. of shoots (128), was leaf explant on MS+BAP(0.1 mg/l) which had been subcultured from MS+BAP (2.0 mg/l) medium (Table-3). figure-C
Whereas the micro-shoots from other treatments did not gave the same results in comparison to above treatment. The elongation of shoots and proliferation of nodes were achieved on MS+BAP (0.5 mg/l) medium, produced shoots having nice shoot lenght . In all above treatments after four weeks highest shoot length was 12.1 cm found on the medium MS+BAP(0.5 mg/l) in case of leaf explant but in case of nodal segment this was 13 cm on MS+ BAP(1.0 mg/l)+ Kn (0.5 mg/l), (Table-2). In our studies highest no. of shoot produced in lower concentration of BAP. In present study, the BAP combination was found to be the best for regeneration and multiplication of micro-shoots production. Root induction and proliferation:- After three cycles of multiplication sub-culturing all the in vitro shoots 6-7 cm in length were separated and subculture individually on the medium Half strength and full strength containing auxins in different concentration. Root induction was observed in all treatment. MS+IBA (1.0 mg/l) was found to be the best medium for highest no. of roots (9.2) in case of full strength whereas the least no. roots i.e 4.8 occurred on higher concentration of IBA i.e. MS+IBA (2.0 mg/l). Half strength MS + IBA (1.5 mg/l) gave best response i.e. 13.1 roots. However, half strength MS medium IBA (1.0 mg/l) gave 12.5 no. of roots. Higher concentration of auxins did not gave the better responses. Highest root length i.e. 6.4 and 5.8 cm was achieved on half strength MS +NAA (1.0 mg/l) and half strength MS+NAA (0.5 mg/l) respectively. NAA combination with half strength MS medium was superior over IBA combination with half strength MS medium in case of root length. In our studies, very fast rooting was achieved on half strength MS + NAA (0.5 mg/l) figure-D within a week with nice root length. Thick rooting was found on Half strength MS+ IBA (1.0 mg/l) figure-F and more thick, branched rooting was found on half strength MS+IBA (1.5 mg/l) figure-E. Thus in present study NAA is best suitable for very fast rooting while IBA was suitable for thick and branched rooting (Table- 4).
RAPD Analysis:-The RAPD analysis was performed with mother plants and in vtro generated plants using six different RAPD primers. Image show the profiles generated with primers GBP-01, GBP-02, GBP-03, GBP-04, GBP-05 and GBP-06 respectively against DNA from both type of plants. For a given primer, the profiles of DNA fragments found for sample A (mother plant) was similar to sample B (in vitro generated plants). With each of the primers, DNA fragment length polymorphisms were observed; several amplicons were of identical size and hence were considered to be the same allele. The reproducibility of the RAPD profiles was confirmed by generating identical DNA fragments from re-extracted DNA, as well as ultra diluted (1:100) RAPD product solution generated from previous reactions. These results suggest the absence of intraspecific polymorphism among two samples for RAPD analysis. The RAPD profiles using GBP-01, GBP-02, GBP-03, GBP-04, GBP-05 and GBP-06 primers were not able to detect DNA polymorphisms among both samples in this study. However primer GBP-01 generated a single, low-intensity, polymorphic band but the similarity coefficient depicted by this primer was found to be 0.95. The overall mean genetic distance between the two samples was computed to be 0.0083. The mean similarity coefficient was found to be 0.9917. From this result it may be concluded that the two samples in question are highly similar in terms of their genetic complexity as deduced by DNA fingerprinting experiments using a definite set of RAPD primers.
DISSCUSSION
In various explants cultured on growth regulator free MS medium did not exhibit any regeneration response however, when MS medium was supplemented with diffenent cytokinins alone or in combination, multiple shoots formation occurred within three weeks. In all treatments best response occurred in leaf explant which gave highest no. of micro-shoots with 100% response. Many earlier reports on shoot bud initiation in Bacopa monnieri plant supports our studies (Tiwari et al., 1998, 2000, 2001, Srivastva and rajni 1999). They reported the leaf explant was more responsive than other explants and gave best results. Among two cytokinins BAP showed better results than Kn. In our studies, MS+BAP (1.5mg/l) gave 23.7 shoots whereas MS+Kn (1.5 mg/l) gave only 18.9 shoots in case of nodal segment (Table-1). Tiwari et al., (1998, 2001) reported that shoot bud induction on different cytokinins & TDZ supplemented medium. He reported that BA (8.9 DISSCUSSION In various explants cultured on growth regulator free MS medium did not exhibit any regeneration response however, when MS medium was supplemented with diffenent cytokinins alone or in combination, multiple shoots formation occurred within three weeks. In all treatments best response occurred in leaf explant which gave highest no. of micro-shoots with 100% response. Many earlier reports on shoot bud initiation in Bacopa monnieri plant supports our studies (Tiwari et al., 1998, 2000, 2001, Srivastva and rajni 1999). They reported the leaf explant was more responsive than other explants and gave best results. Among two cytokinins BAP showed better results than Kn. In our studies, MS+BAP (1.5mg/l) gave 23.7 shoots whereas MS+Kn (1.5 mg/l) gave only 18.9 shoots in case of nodal segment (Table-1). Tiwari et al., (1998, 2001) reported that shoot bud induction on different cytokinins & TDZ supplemented medium. He reported that BA (8.9 µM) was more effective than Kn in shoot bud regeneration but in our studies, we did not use TDZ to minimize the cost of protocol. Banerjee and Shrivastava (2008), reported that MS+Kn 1.5 gave 12 shoots MS+BA (1.5 mg/l) gave only 11 shoots thus the condition is just reverse in this study. In many earlier reports, TDZ has been used more frequently for shoot regeneration in Bacopa monnieri (Tiwari 2001, Antony et al., 2010, mok et al., 2005, Thomas and Katterman 1986). In our studies very higher concentration of PGRs produced less no. of shoots. This has been confirmed by earlier reports ( Tiwari et al.,1998, Banerjee and Shrivastava 2008, Shrivastava and rajni1999). MS+BAP (0.1 mg/l) was proved best medium for shoot multiplication and differentiation as it gave highest no .of shoots i.e. 128 using leaf explant but in case of nodal segment it gave 92 shoots . This concentration increased the no. of shoots as well as shoot length. In earlier studies Tiwari et al., (2006) reported 81 and 98 shoots from inter node and leaf respectively. In other report shoot clump obtained from shoot induction medium were sub-cultured in the multiplication medium containing lower concentration of BAP, produced 135 shoots from leaf explants (Antony et al., 2010). On sub-culturing when we used lower concentration of cytokinins more no. of shoots produced. Praveen et al., (2009), proves that the Kn is better than the BAP for formation of multiple shoots in bacopa monnieri, he used MS+Kn (2 mg/l) which gave hightest no. of shoots from leaf explants. Addition of auxin (NAA, IAA and IBA) to cytokinins had significantly enhanced the frequency of shoot induction.NAA produced more shoots over IAA/ IBA when either added to cytokinins (Antony et al., 2010). Many other reports suggests the use of auxins to cytokinins for better shoot production ( Dimitrov et al., 2003, Skala and Wysokinska 2004). In present study we used BAP (1.5 mg/l) + NAA (0.5 mg/l) and BAP (1.5 mg/l)+ IBA(0.5 mg/l) for shoot formation, they produced 29.4 and 26.3 shoots respectively. Present study validates the 100% response in rooting of bacopa monnieri plant. Tiwari et al., (2000) reported that the highest rate of rooting (90%) on MS full strength medium containing 2.46 mM IBA. Tiwari et al., (1998) reported that higher no.of roots in bacopa produced on IBA medium than NAA medium however Banerjee and Shrivastava (2008) reported that NAA showed more no.of roots. Half strength MS medium with IBA/ NAA produced more roots in Bacopa monnieri than full strength MS medium (Antony et al., 2010), in Tylophora indica (Faisal and Anis 2005), in Rhodiola fastigiata (Liu et al., 2006). In our investigations , MS half strength medium gave 100% response ( no. of roots 13.1) while MS full strength medium gave less no. of roots i.e. 9.3 thus MS half strength medium was superior to MS full strength medium producing more no. of roots and this is supported by many earlier reports. IBA (1.5 mg/l) gave best results in present study however in other studies, Antony et al., (2010) reported that IBA was superior to IAA for the root induction of Bacopa monnieri. Similar results found IBA (4.9 µM) gave higher no. of roots in this plant (Tiwari 2001). In this study it was proved that the DNA profiles of mother plants and the in vitro generated plants were same and did not show any polymorphism. This clearly indicates that the micropropagated plants which are the clones of the mother plants having same DNA or same genetic makeup because they did not showing any genetic diversity in RAPD analysis. This method is very easy,quick, less time taking and very efficient . RAPD has been proven to be a suitable molecular technique to detect the variation that is induced or occurs during in vitro International Journal of Current Research and Review www.ijcrr.com Vol. 03 issue 09 September 2011 173 regeneration of plant species (Shu et al.2003). a number of workers done this method for several plants to identify the genetic makeup and detect polymorphism as Curcuma amada (Prakash et al. 2004), Plumbago zeylanica (Rout and Das 2002), Drosera anglica and Drosera binata (Kawiak and Lojkowska 2004), Musa paradisiacal (Venkatachalam et al. 2007), Zingiber officinale (Rout et al. 1998), Curcuma longa (Selvi et al.2002).
CONCLUSION
MS medium augumented with different PGRs , explants of Bacopa monnieri showed positive response in the sense of microshoots and roots production. When MS medium supplemented with BA (1.5), highest no. of shoots (23.7) developed. In our study BA proved to be better than Kn in shoot initiation treatments . Whereas Cytokinins and auxins treatments BA(1.5)+ NAA(0.5) showed best results i.e. 29.4 no. of shoots produced. But in comparison to NAA , IBA produced less no. of shoots . Thus NAA is superior to IBA combinations. However, when we used two cytokinins BA & Kn, BA showed better response than the Kn but one thing is common in both treatments i.e. Lower concentrations of both gave satisfactory results than the higher ones. In case of shoot multiplication treatments, BA(0.1) gave excellent results i.e. 128 no. of shoots produced by leaf explants when subcultured from BA(2.0) (shoot initiation medium). Similarly BA(0.5) gave 111 shoots. Thus here , leaf explants and lower concentrations of BA gave best respone. But N.S. Explants also gave satisfactory results i.e. 92.4 no. of shoots developed on BA(0.1) after subculturing from BA(1.5)+ NAA (0.5)(shoot induction medium).In case of rooting, MS ½ +IBA(1.5) produced higher no. of roots i.e.13.1 and MS +IBA(1.0) gave 9.2 no. of roots but MS ½ + NAA (0.1)gave 11.8 no. of roots & MS+NAA(0.1) gave 7.9 no. of roots. Therefore IBA and MS½ medium showed good results than NAA and MS medium respectively in rooting.The plants were successfully acclimatized in green-house at 95-100% survival rates.
Plant samples analyzed using RAPD techniques in this study suggest that the pattern obtained for Sample A was similar with respect to RAPD banding patterns of other sample B analyzed in the experiment.The RAPD profiles using GBP-01 to GBP-06 primers were not able to detect DNA polymorphisms among these samples . However primer GBP-01 generated a single, low-intensity, polymorphic band but the similarity coefficient depicted by this primer was found to be 0.95. The mean similarity coefficient was found to be 0.9917. From this result it may be concluded that the two samples in question are highly similar in terms of their genetic complexity as deduced by DNA fingerprinting experiments using a definite set of RAPD primers. Since Bacopa monnieri is being indiscriminately exploited because of its great medicinal valueand urgent methods for its replenishment and cultivation, preferably through tissue culture , bocome highly desirable.The methodology adopted can ensure a regulate supply of Bacopa monnieri plant to pharmaceutical industries.
ACKNOWLEDGEMENT
The first author is thankful to the Prof. Moinuddin Khan (Dean ) School of life science, Singhaniya University, for providing the necessary facilities and giving valuable suggestions and time.



References:
1. Ahmad, R.U. Medicinal plants used in ISM – their procurement, cultivation, regeneration and import/export aspects: a report. In: JN Govil, VK Sing and S Hashmi (editors). Medicinal Plants: New International Journal of Current Research and Review www.ijcrr.com Vol. 03 issue 09 September 2011 174 Vistas of Research, Part I. Today and Tomorrow Publishers,New Delhi, India. 1993; pp.221–258.
2. Ali, G., Srivastava, P.S., Iqbal, M. Morphogenic and biochemical responses of Bacopa monnieri cultures to zinc toxicity. Plant Science 1999;143:187–193.
3. Banerjee, Meenakshi and Shrivastava, Sarika. An improved protocol for in vitro multiplication of Bacopa monnieri (L.) World J Microbiol Biotechnol 2008; 24:1355–1359
4. Ceasar, Antony,S., Maxwell,Lenin,S., Prasad, Bhargav,K., Karthigan,M., Ignacimuthu, S. Highly efficient shoot regeneration of Bacopa monnieri (L.) using a two-stage culture procedure and assessment of genetic integrity of micropropagated plants by RAPD Acta Physiol Plant 2010;32:443–452
5. Dhanasekaran, M., Tharakan, B., Holcomb, L.A., et al., "Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera." Phytother Res. 2007; 21:965- 969.
6. Dimitrov, B., Tashera, K., Zagorska, N., Evstatiera, L. In vitro cultivation of Rhodiola rosea L. Gene Breed 2003;32:3–6
7. Elangovan, V., Govindasamy, S., Ramamoorthy, N., Balasubramanian, K. In vitro studies on the anticancer activity of Bacopa monnieri. Fitoterapia 1995;66:211–215.
8. Faisal, M., Anis, M. An efficient in vitro method for mass propagation of Tylophora indicia. Biol Plant 2005; 49:257–260
9. George, S., Geetha, S.P., Balachandran, I., Ravindran, P.N. Micropropagation and in vitro conservation studies in Bacopa monnieri (L.) Pennell—The MemoryPlus Plant. In: Abstracts of the 26th Annual Meeting of the Plant Tissue Culture Association of India and National Symposium on Biotechnology for a Better Future. St. Aloysious College, Mangalore, India,15–17 January 2004. p.32.
10. Jayanthi, M., Mandal, P.K. Plant regeneration through somatic embryogenesis and RAPD analysis of regenerated plants in Tylophora indica (Burm. F. Merrill.). In Vitro Cell Dev Biol Plant 2001;37:576–580
11. Kawiak, A.A., Lojkowska, E. Application of RAPD in the determination of genetic fidelity in micropropagated drosera plantlets in vitro cell. Dev Biol Plant 2004; 40:592– 595
12. Liu, HJ., Xu, YAN, Liu, Y.-J., Liu, C.-Z., Plant regeneration from leaf explants of Rhodiola fastigiata. In Vitro Cell Dev Biol Plant 2006; 42:345–347
13. Mathur, S., Kumar, S. Phytohormone selfsufficiency for regeneration in the leaf and stem explants of Bacopa monnieri. Journal of Medicinal and Aromatic Plant Sciences 1998; 20:1056–1059.
14. Mathur, S. Study of the genetic variability in the traditional Indian medicinal plants Centella asiatica and Bacopa monnieri towards their domestication. PhD Thesis, Lucknow University, Lucknow, India 1999.
15. Misra, Y., Rana, P.K., Rani, A., Chawhaan, P.H. In vitro organogenesis from leaflet explants in Bacopa monnieri Linn. – An important medicinal plant. Journal of NonTimber Forest Products 2003;10:212–217.
16. Mok, M.C., Mok, D.W., Amstrong, D.J., Shudo, K., Isogai, Y., Okamanto, T. Cytokinin activity of N-phenyl-N0-1, 2, 3- thiadiazol-5- urea (thidiazuron). Phytochemistry 2005; 21:1509–1511
]17. Murashige, T., Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 1962; 15: 473 – 497 International Journal of Current Research and Review www.ijcrr.com Vol. 03 issue 09 September 2011 175
18. Praveen, N., Naik, P.M., Manohar, S.H., Nayeem, A. and Murthy, H.N. In vitro regeneration of brahmi shoots using semisolid and liquid cultures and quantitative analysis of bacoside A. Acta Physiol. Plant 2009; 31: 723-728
19. Prakash, S., Elangomathavan, R., Seshadri, S., Kathiravan, K., Ignacimuthu, S. Efficient regeneration of Curcuma amada Roxb plantlets from rhizome and leaf sheath explants. Plant Cell Tissue Organ Cult 2004; 78:159–165
20. Rajani, M., et al. "Brahmi (Bacopa monnieri (L.) Pennell) – A Medhya Rasaayana Drug of Ayurveda" in Ramawat, K. G., Ed. Biotechnology of Medicinal Plants: Vitalizer and Therapeutic Enfield, New Hampshire: Science Publishers, Inc.2004.
21. Roodenrys, S ., Booth, D., Bulzomi, S., Phipps, A., Micallef, C., Smoker, J.?Chronic Effects of Brahmi (Bacopa monnieri) on Human Memory?. Neuropsychopharmacology (Wollongong) 2002;27, 2:279–281.
22. Rout, G.R., Das, P. An assessment of genetic integrity of micropropagated plants of Plumbago zeylanica by RAPD markers. Biol Plant 2002; 45:27–32
23. Rout, G.R., Das, P., Goel, S., Raina, S.N. Determination of genetic stability of micropropagated plants of ginger using random amplified polymorphic DNA (RAPD) markers. Bot Bull Acad Sin 1998; 39:23–27
24. Selvi, D.N., George, L., Eapen, S. Micropropagation and field evaluation of micropropagated plants of turmeric. Plant Cell Tissue Organ Cult 2002; 68:143–151
25. Singh, H.K., Rastogi, R.P., Sriman, R.C., Dhawan, B.N. Effect of bacoside A and B on avoidance response in rats. Phytother Res 1988; 2:70–75
26. Skala, E., Wysokinska, H. In vitro regeneration of Salvia nemorosa L. from shoot tips and leaf explants. In Vitro Cell Dev Biol Plant 2004;40:596–602
27. Srivastava, N. and Rajani, M. Multiple shoot regeneration and tissue culture studies on Bacopa monnieri (L.) Pennell. Plant Cell Reports 1999;18:919–923.
28. Stough, C., Lloyd, J., Clarke, J., Downey, L.A., Hutchison, C.W., Rodgers, T., Nathan, P.J. The chronic effects of an extract Bacopa monniera (Brahimi) on cognitive function in healthy human subjects. Psychopharmacology 2001;156, 4:481–484
29. Stough, C., Downey, L.A., Lloyd, J. et al. "Examining the nootropic effects of a special extract of Bacopa Monniera on human cognitive functioning: 90 day double-blind placebo-controlled randomized trial." Phytother Res. 2008; 22:1629-1634.
30. Tejavathi, D.H. and Shailaja, K.S. Regeneration of plants from the cultures of Bacopa monnieri (L.) Pennell. Phytomorphology 1999; 49, 4:447–452.
31. Thomas, J.C. and Katterman, F.R. Cytokinin activity induced by thidiazuran. Plant Physiol 1986; 81:681–683
32. Tiwari, V., Deo, Singh, B., Nath, Tiwari, K. Shoot regeneration and Somatic embryogenesis from different explants of Brahmi (Bacopa monniera (L.) Wettst.). Plant Cell Rep 1998; 17:538–543
33. Tiwari, V., Tewari, K.N., Singh, B.D. Suitability of liquid cultures for in vitro multiplication of Bacopa Monniera (L.) Wettst. Phytomorphology 2000; 50:337–342
34. Tiwari, V., Tewari, K.N., Singh, B.D. Comparative studies of cytokinin on in vitro propagation of Bacopa monniera. Plant Cell Tissue Organ Cult 2001;66:9–16 International Journal of Current Research and Review www.ijcrr.com Vol. 03 issue 09 September 2011 176
35. Tiwari, V., Tewari, K.N., Singh, B.D. Shoot bud regeneration from different explants of Bacopa monniera (L.) Wettst. by trimethoprim and bavistin. Plant Cell Rep 2006;25:629–635
36. Tripathi, Y.B., Chaurasia, S., Tripathi, E., Upadhyaya, A., Dubey, G.P. Bacopa monnieri Linn. as an antioxidant: mechanism of action. Indian Journal of Experimental Biology 1996; 34:523–526.
37. Venkatachalam, L.E., Sreedhar, R.V., Bhagyalakshmi, N. Micropropagation in banana using high levels of cytokinins does not involve any genetic changes as revealed by RAPD and ISSR markers. Plant Growth Regul 2007; 51:193–205
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License