IJCRR - 4(3), February, 2012
Pages: 108-114
Print Article
Download XML Download PDF
CHARACTERIZATION OF FATTY ACIDS IN MELIA AZEDARACH L. SEED OIL
Author: R. K. Bachheti, Himanshu Dwivedi, Vikas Rana, Indra Rai Archana Joshi
Category: General Sciences
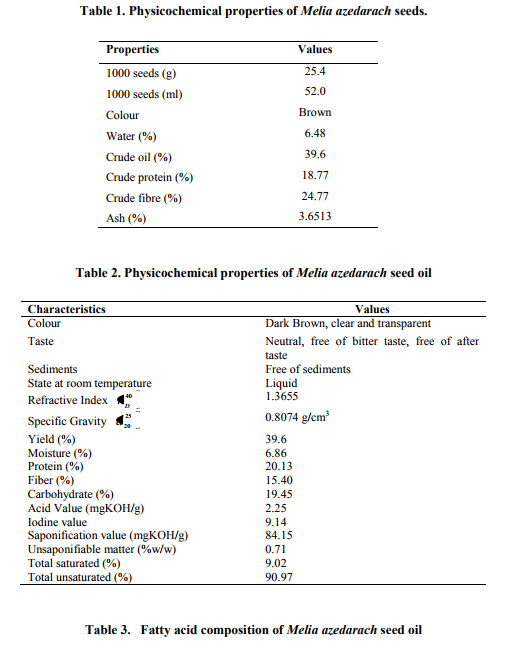
Abstract:Physical and chemical characteristics of oil extracted from Melia azedarach L. seeds are presented in this work. The results indicate that Melia azedarach seeds constituted 39.6% crude oil. The specific gravity, refractive index, saponification value, and unsaponifiable matter content of Melia azedarachseed oil were found to be 0.80 g/cm3, 1.36 40 D n , 84.15 (mg KOH/g) and 0.71 (%w/w) respectively. Elemental analysis of oil by AAS and Titration method shows that it contains Ca (1230 mg/100g), Mg (990 mg/100g), K (121 mg/100 g), Zn (3.12 mg/100 g), Mn (3.4 mg/100g), Fe (19.52 mg/100g) and P (213 mg/100g). The extracted oil was methyl-esterified and analyzed by GC and GC-MS. The major fatty acids reported here, in Melia azedarach seed oil, are Palmitic acid (5.68%), Linoleic acid (74.57%), Oleic acid (16.39%), Stearic acid (3.33%). This work might be useful for developing applications for Melia azedarach L. seed oil.
Keywords: Melia azedarach, seed, oil.
Full Text:
INTRODUCTION Melia azedarach L. (Sapindales: Meliaceae), know as Chinaberry or Persian lilac tree, is a deciduous tree native to northwestern India and has long been recognized for its medicinal and insecticidal properties but yet to be properly analyzed. This tree typically grows in the tropical and subtropical parts of Asia, but nowadays it is also cultivated in other warm regions of the world because of its considerable climatic tolerance1 . It has been cultivated since the sixteenth century, chiefly for ornamental purposes and has become naturalized in most tropical and subtropical countries2 . In Traditional Chinese Medicine, the plant is used as an antiparasitic and antifungal agent, but many of its constituent compounds have been found to exhibit a wide range of other biological properties3-10 . In addition, a number of potent pharmaceutical limonoids and triterpenoids have been isolated from fruits and bark11 . The cytotoxic property of limonoids is extensive and recent efforts are designed to investigate the cellular and molecular mechanisms by which such effects are exerted in the tumorigenic cell lines12 . Although the fruits are the poisonous part of the tree, they have been used traditionally for the treatment of a variety of diseases, specially dermatitis and rubella11 . The bark and rootbark mainly contain tetracyclic triterpenoids, as well as flavones and anthraquinones, etc. Pharmacological studies indicate that the bark, fruit, seed and leaf have the effects of expelling parasites, suppressing bacteria and anti-virus, etc13 . In some parts of Tamil Nadu, India the decoction of the leaves of the tree is used under traditional system of medicine to cure the problem of dysmenorrhoea (pain and discomfort during menstruation). They gave the suffering patients the decoction of the leaves of Persian Lilac tree, `Malaivembu' in Tamil, Melia azedarach and it is known to cure the conditions very fast. The object of this study was therefore to extract oil from Melia azedarach seeds and assessment of the physical and chemical characteristics of the oil as a prelude to an investigation into the scientific basis for its best end uses. MATERIALS AND METHODS Collection of plant materials The seeds of Melia azedarach L. are small (about 6-7 mm long) and enclosed in a thick hard bony endocarp commonly known as stone. The fruits of Melia azedarach were collected from Sahestra Dhara road, Dehradun in month of December 2010. The fruit were cleaned and stones were separated. The stones were broken manually to obtain seeds. The seeds were air dried in the shade for few days and kept in colour bottles until analyzed. Extraction of seed oil 100 gm of seeds were grounded into powder form with high speed blender and dried in an air circulating oven at 50oC for 1 h. Oil was extracted from the dried grounded seeds with petroleum ether (boiling point 60-80oC) using a Soxhlet extractor. The solvent was distilled off at 80oC. Oil content was calculated on the basis of dry seeds weight and expressed in g/100g. Analysis of seed oil Oil density was determined picnometrically, Refractive index was determined at 25°C with Abbey Refractometer, viscosity was determined by Ostwald method14. The oil extracted from the seeds was assessed for various chemical properties. Standard methods described by Association of Official Analytical Chemists15 were used for the determination of moisture, crude fibre and ash contents of the seed samples. Physical and chemical analyses of the extracted oil were carried out by using AOAC methods15. Iodine value was determined using Wij‘s method as reported in AOAC methods15. The procedures of Egan et al. 16 were adopted for the estimation of saponification values, unsaponifiable matter content and acid value of the oil sample. Protein content in seeds and oil sample was determined using microKjeldhal method as described by Allen and Quarmby17 . A factor of 6.25 was adopted for protein content estimation. Carbohydrate content was determined by colorimetric method17 . The metal composition Zinc, Iron, and Manganese of the seeds were determined by using an Atomic Absorption Spectrophotometer (Model Varian 240FS+GTA120), after acid digestion. Calcium and magnesium was determined by complexometric titration with 0.1M EDTA, by using Erichome black T indicator and calculated. Phosphorus was determined by the precipitation of phosphorus in the form of phospho molybdate by using the reagent ammonium molybdate. Precipitate was filtered from asbestos, then residue obtained was taken in Conical Flask and dissolved in 0.1 M NaOH and titrate with 0.1 M HCl by using indicator Phenolphthalein. Potassium was determined by flame photometer model No. ESICO 1381 by using the reference standard (Merck) and calculated on the basis of reading and dilution of the sample. GC and GC-MS analysis The Fatty acids were derivatized by using the boron trifluoride method as described by Hisil18. Samples were injected as 2 µl into a Nucon model 5700 equipped with 10% DEGS (Diethylene Glycol Succinate) + 1% H3PO4 constant phase, a flame ionization detector (FID) and chromosorb G (100/120 mesh) support matter, internal diameter (2mm) and stainless steel (190 cm) column. Column temperature was programmed from 70°C to 200°C with the increasing rate of temperature 6°C/Minute. Injector and detector temperatures were set at 225°C. Nitrogen (N2) (25 ml/min) was used as the carrier gas.
Hydrogen (40ml/min) and Air (60ml/min) were used as burnt and dry gas respectively. Fatty acid methyl esters were identified by comparison with fatty acid internal standards, Individual fatty acid concentration was expressed as percent RESULTS AND DISCUSSION The seeds of Melia azedarach were collected in the month of December, 2010 from Dehradun (Uttarakhand), India. The seeds were dark brown in colour and evaluated for physical properties. Analysis results of seeds are given in table-1. Seeds are rich in protein, oil and fibre. Oil extracted (yield; 39.6 %w/w) from Melia azedarach seeds is dark brown in colour and free from sediments. It is liquid at room temperature (27± 2OC). It contains 20.13% protein, 19.45% carbohydrates and 15.40% crude fibre. The physico-chemical properties of Melia azedarach seed oil is given in table-2. The results of GC (figure-1) and GCMS analysis of oil is listed in table-3 and showed that the oil contain both saturated (9.0226%) and unsaturated fatty acids (90.9774). The main acids present in the oil were Palmitic acid (5.68%), Linoleic acid (74.57%), Oleic acid (16.39%), Stearic acid (3.33%). Acid value is an indicator for edibility of oil and suitability for industrial use. Melia azedarach seed oil has an acid value 2.25. This falls within the recommended codex of 0.6 and 10 for virgin and non virgin edible fats and oil respectively19. The iodine value of Melia azedarach oil is 9.14 which indicate that it is drying oils. The low iodine value in this study indicate the oil contain low level of polysaturated fatty acids. The seed oil studied have a significant saponification value 84.15, the high saponification value recorded for the seed oil suggested that the oil contain high molecular weight fatty acid and low level of impurities. This is evidence that the oil could be used in soap making industry20, 21 . The main chemical component of the fatty acids in Melia azedarach is Linoleic acid. Linoleic acid is the essential amino acid, and be supplied to the human beings only by food sources. It helps low blood pressure in hypertensive patients, and also be useful to protect human cardiac system22, 23. It is used for manufacturing margarine, shortening, and salad and cooking oils as well as soaps, emulsifier, and quick drying oils24. The other main chemical component is Oleic acid, it reaches 16.39%. Oleic acid is the most abundant fatty acid in human adipose tissue25 . Oleic acid may hinder the progression of adrenoleukodystrophy (ALD), a fatal disease that affects the brain and adrenal glands26 . Oleic acid is also responsible for the hypotensive effects of olive oil27 . As an excipient in pharmaceuticals, oleic acid is used as an emulsifying or solubilizing agent in aerosol products28. In Melia seed oil the palmitic acid and stearic acid contributed 5.68 and 3.33% respectively. Palmitic acid is used in the manufacture of soaps, candle, cosmetic formulations, food grade additives, waterproofing agents, lube oils, and non drying oils (surface coatings). Whereas the presence of stearic acid in Melia seed oil indicates the potential use of oil for pharmaceutical preparations, dietary supplements, oil pastels, soaps, food packaging, deodorant sticks, toothpaste and softening rubber29, 30 . The mineral composition of Melia azedarch is summarized in table-4. It is rich in Calcium, Magnesium, Potassium and Iron which make it quite suitable as edible and commercial oil. Considering the results obtained in this preliminary study, it is noticeable that the seeds oil had a high content of linoleic acid and oleic acid and also has a healthy composition for nutrition. It turned out that Melia azedarach could be good source of natural oil rich in Linoleic acid and Oleic acid. This work might be useful for exploring the applications of Melia azedarach seeds and its oil. Further by cultivation and breeding of capers plants regularly, a more productive quality raw matter would be obtained.
ACKNOWLEDGEMENT Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed
References:
1. WAC, World Agroforestry Centre, Agroforestry Tree Database, A tree species reference and selection guide [http://www.worldagroforestry.org/sea/Pr oducts /AFDbases/af/asp /SpeciesInfo.asp?SpID=1141] (Accessed September 23, 2011).
2. Hadjiakhoondi A, Vatandoost H, Khanavi M, Sadeghipour-Roodsari R, Vosoughi M, Kazemi M, Abai M R., Fatty acid composition and toxicity of Melia azedarach L. fruits against malaria vector Anopheles stephensi. Iran. J. Pharma.Sci. 2006; 2(2): 97-102.
3. Isman M B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006; 51: 45-66.
4. Akhtar Y, Yeoung Y R, Isman M B. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars,Trichoplusia ni and Pseudaletia unipuncta. Phytochem. Rev. 2008; 7 (1): 77-88.
5. Carpinella M C, Ferrayoli C, Valladares G, Defago M, Palacios S. Potent limonoid insect antifeedant from Melia azedarach. Biosci. Biotechnol. Biochem. 2002; 66 (8): 1731-1736.
6. Carpinella M C, Ferrayoli C G, Palacios S M. Antifungal synergistic effect of scopoletin, a hydroxycoumarin isolated from Melia azedarach L. fruits. J. Agric. Food Chem. 2005; 53 (8): 2922-2927.
7. Carpinella M C, Giorda L M, Ferrayoli C G, Palacios S M. Antifungal effects of different organic extracts from Melia azedarach L. on phytopathogenic fungi and their isolated active components. J. Agric. Food Chem. 2003; 51 (9): 2506- 2511.
8. Coria C, Almiron W, Valladares G, Carpinella C, Ludueña F, Defago M, et al. Larvicide and oviposition deterrent effects of fruit and leaf extracts from Melia azedarach L. on Aedes aegypti (L.) (Diptera: Culicidae). Bioresource Technol. 2008; 99 (8): 3066-3070.
9. Kamaraj C, Rahuman A A, Bagavan A, Mohamed M J , Elango G , Rajakumar G, et al. Unit Ovicidal and larvicidal activity of crude extracts of Melia azedarach against Haemonchus contortus (Strongylida). Parasitol. Res. 2010; 106 (5): 1071-1077.
10. Ntalli N G, Menkisoglu-Spiroudi U, Giannakou I. Nematicidal activity of powder and extracts of Melia azedarach fruits against Meloidogyne incognita. Ann. Appl. Biol. 2010; 156 (2): 309-317.
11. Alché L E, Ferek G A, Meo M, Coto C E, Maier M S. An antiviral meliacarpin from leaves of Melia azedarach L. Z. Naturforsch. 2003; 58c (1/2): 215-219.
12. Ntalli N G, Cottiglia F, Bueno C A, Alché L E, Leonti M, Vargiu S, et al., Cytotoxic tirucallane triterpenoids from Melia azedarach fruits. Molecules. 2010; 15 (9): 5866-5877.
13. Anonymous. Hong Kong Jockey Club Institute of Chinese Medicines, Encyclopedia on Contemporary Medicinal Plants, Melia azedarach (Chinaberry-tree) [http://www.hkjcicm.org/cm_database/pl ants/detail_e.aspx?herb_id=35] (Accessed August, 25, 2011).
14. Boži? J S, Ogrin T. Viscosity. [http://www.standardbase.hu/tech/SITech Visc.pdf.] (Accessed July, 12, 2011). 15. A.O.A.C. Official Methods of Analysis 14th Edn. Association of Official Analytical Chemists. Washington D. C. 1990; 14th Edn.: pp. 801-805.
16. Egan H, Kirk R S, Sawyer R. Pearson‘s Chemical Analysis of Foods. 8th Edn. London: Churchill Livingstone Publishers; 1981: pp. 507-547.
17. Allen S E, Quarmby C., Organic Constituents. In: Allen S E, editor. Chemical Analysis of Ecological Materials, London: Blackwell Scientific Publications; 1989; p.160-200.
18. Hisil Y. Instrumental Analysis Technique. Izmir, Turkey: Ege Univ. Engineering Fac. Publ. Nu.55;1989.
19. Ibrahim T A, Dada I B O, Adejare R A. Comparative phytochemical properties of crude ethanolic extracts and physicochemical characteristics of essential oils of Myristical fragrans (Nutmeg) seeds and Zingiber officinate (ginger) roots. Electronic Journal of Enviornment, Agriculture and Food Chemistry. 2010; 9(6): 1110-6.
20. Kirsehenbauer H G. Fats and Oil: An Outline of their Chemistry and Technology. 2nd edn. New York: Reinhold Publ Corp. 1965; p. 160-161.
21. Akanni M S, A-dekunle S A, Oluyemi E A. Physio-Chemical properties of some non-conventional oil seed. J. Food Technol. 2005; 3:177-181.
22. Zhen L I, Yang De-po. Structure-effect relationship of conjugated linoleic acid and its molecular pharmacology research progress. J. Int. Pharmaceutical Res. 2007; 34(1): 26-30.
23. Whigham L D, Cook M E, Atkinson R L. Conjugated linoleic acid: implications for human health. Pharmacol Res. 2000; 42(6): 503-510.
24. Ukalina, O G, Ifechukwude N M. Characterization of the fatty acids of Gardenia jasminoide flower from port Harcourt, Nigerian. International Journal of Academic Research. 2011; 3 (3): 534- 538.
25. Kokatnur M G, Oalmann M C, Johnson W D, Malcolm G T, Strong J P. Fatty acid composition of human adipose tissues from two anatomical sites in a biracial community. Am. J. Cli. Nutr., 1979; 32 (11): 2198–205.
26. Rizzo W B., Watkins P A, Phillips M W, Cranin D, Campbell B, Avigan J. Adrenoleukodystrophy: oleic acid lowers fibroblast saturated C22-26 fatty acids. Neurology. 1986; 36(3): 357-61.
27. Terés S, Barceló-Coblijn G, Benet M, Alvarez R, Bressani R, Halver Je, et al. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Nat. Acad. Sci. U.S. A. 2008; 105 (37): 13811–6.
28. Smolinske S C. Handbook of Food, Drug, and Cosmetic Excipients. New York: CRC Press; 1992: p. 247–248.
29. Okieimen F E, Eromosele C O. Fatty acid composition of Khaya senegalensis. Bioresource Technol. 1999; 69: 279-280.
30. Wootthikanokkhan J, Tunjongnawin P. Investigation of the effect of mixing schemes on cross link distribution and tensile properties of natural acrylic rubber blends. P

|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License