IJCRR - 4(3), February, 2012
Pages: 32-45
Print Article
Download XML Download PDF
BLOOD GLUCOSE CONCENTRATION - A KEY TO FIX THE EFFECTIVE DOSE FOR HERBAL ANTIDIABETIC DRUGS USING RAT MODEL
Author: R.Kannadhasan, S.Venkataraman
Category: Healthcare
Abstract:Sedimental Extract of Tinospora cordifolia (SETc), with no mortality rate at the maximum of 2000mg/kg/p.o., acute dose was found to show maximum number of deaths on chronic treatment in the mid of 28 days repeated oral toxicity study. A trial made on SETc at incremental doses starts from the minimum of 250 - 1000 mg/kg/p.o., were then subjected to modified IDF procedure, to study their safer therapeutic margin. Sprague dawley rats were made diabetic with streptozotocin (45mg/kg/i.p.) and the OGTT procedure was performed on those diabetic rats, fasted around 16 hours prior to the commencement of IDF study. Starting from the 30th min after glucose load (1g/kg/p.o.), the incremental doses of SETc, from the minimum of 250mg/kg/p.o., to the maximum of 1000 mg/kg/p.o., were administered to each group. The reduced blood glucose levels from each group were analyzed and derived by means of AUC and thereby safer therapeutic and effective dose of the test drug was fixed. The onset of action of all the doses of the SETc originates
from the 60th min of the drug administration and showed the biological responses in a concentration dependant manner. Based on the IDF, AUC and EDF data's, it was found to be very clear that the dose of 1000 mg/kg/p.o., of SETc was found to underlie the safer therapeutic margin than the other doses. This evidences that the application of this modified method would be a valuable tool for finding safer therapeutic marginal dose using BGC as a key factor.
Keywords: Sedimental Extract of Tinospora cordifolia (SETc), Oral Glucose Tolerance Test (OGTT), Blood Glucose Concentration (BGC), Incremental dose finding (IDF), Area under the Curve (AUC), Effective dose finding (EDF), Streptozotocin (STZ) induced diabetic rats.
Full Text:
INTRODUCTION
The major hindrance to the use of the herbal preparation in clinical practice is due to the lack of preclinical data for understanding the safety and efficacy of the drugs. For the evaluation of various forms of oral herbal preparations, instead of their treatment profile there must be a need of strong evidence, for its safer therapeutic index. Uncertainty, of dose fixation during preclinical toxicity studies also rules a part. Since it‘s in need and in deed to fix the effective therapeutic dose of those herbal preparation which would be safer enough with good therapeutic outcome, for long term therapy likely from fluctuating blood glucose levels in diabetics. So it necessitates a modified protocol along with statistical approach, the dose response effect of oral antidiabetic agents in animals could be studied.
AUC has a number of important uses in pharmacology, biopharmaceutics and pharmacokinetics. Through biochemical and hematological parameters, the bio equivalency or bioavailability studies of a compound could be analyzed by comparing its AUC values [1]. But here, the measurement of AUC after administration of an herbal product plays an important role in fixing safer therapeutic dose. Since its diabetic case, the study of alterations in the blood glucose concentration levels were found to be quite worthy to give enough surveillance to analyze the AUC which necessitates its role over preclinical evaluation of a drug dose. The incremental dose finding [2] method adopted to study the dose response relationship with AUC of the herbal antidiabetic agent using linear regression analysis. Based on the priority of work done in diabetes and ease of availability, a random selection of a large, glabrous, succulent, climbing shrub belonging to the family Menispermaceae, namely Tinospora cordifolia, which was used as a folklore medicine in diabetes [3] were made. In accordance to the work being carried out, its planned to make a trial on raw portion of the plant by means of sedimental extraction from the plant stalk which would be supportive than other solvents with particular components. Some of the antidiabetic works in various extracts of Tinospora cordifolia reported as below Aqueous, alcoholic and chloroform extracts of the leaves of T. cordifolia showed hypoglycaemic activity in both alloxan diabetic and normal rabbits at 250 mg/kg of the dose administered [4]. Daily oral administration of an aqueous root extract of T. cordifolia to alloxan diabetic rats for 6 weeks significantly reduced blood glucose levels at 2.5 and 5.0 g/kg, but not 7.5 g/kg. T. cordifolia was more effective than glibenclamide, but less effective than insulin (which restored parameters to near normal values) at lowering blood glucose levels. Instead of their biological action, the rationality for the regression of hypoglycaemic effect at these varying doses was not provided [5, 6, 7and 8]. The current study focused on the dose selectivity, that shows maximum therapeutic efficacy of Sedimental Extract of Tinospora cordifolia (SETc), an oral herbal preparation through incremental dose finding and area under the curve determinations on the OGTT in diabetic rats.
MATERIAL AND METHODS Plant Collection
Tinospora cordifolia collected from Irulars Tribal Women Welfare Society (ITWWS), Thandarai, Thirukazhukundram, a southern forest region of Tamil nadu, India. The stem portions were cut, dried and collected in the month of January 2007 and shade dried for further processessing and studies. The pharmacognositcal identity and authentication was done by Plant Anatomy research Centre, Chennai. A specimen of the plant was kept in the Department of Pharmacology, C.L.Baid Metha college of Pharmacy, Chennai (Specimen No. CLBMCP/102/2005).
Preparation of Plant Extract The dried stem of Tinospora cordifolia, 2 kg was grounded to a coarse powder and soaked in 1000ml of distilled water for a period of 24 hrs, until the active portion to settle down. The top layer was drained in a separate vessel (leaving the debris to filter off) and evaporated in a hot water bath at 100o C, and this portion is considered as water soluble portion. The sedimented portion after removing the water soluble portion was washed for 2-3 times with fresh distilled water. The sedimented extract was admixed with water soluble extract in the ratio of 3:1 to get the final sedimental extract of Tinospora cordifolia (SETc) for screening.
Physico chemical properties Slightly soluble in Ethanol < DMSO and not in other solvents. A fine suspension of the extract was obtained in 0.5 % Sodium Carboxy Methyl Cellulose (Na.CMC). Hence 0.5% Na.CMC suspension of this drug is used for animal experiment. Buff white powder. Bitter taste; Bitterness might be due to admixture of water soluble portion at the final preparation.
Chemicals and equipments Streptozotocin, 97% pure dextrose and Ready-to-use biochemical kits were purchased from Sigma-Aldrich Pvt.ltd, Mumbai. Ascensia One Touch glucometer and strips (Code. No: 3110; 3112) was used to measure the blood glucose concentration.
Animals Male Sprague dawley rats 200-250 gm were purchased from King‘s Institute, Guindy, Chennai. Requirement of animals for this study was authorized by Dr.C.L.Baid Metha College of Pharmacy, CLBMCP/131/IAEC/41 under CPSCEA guidelines. All rats were randomly selected, segregated and acclimatized for a period of 1 week with 12hr day light and 12 hours dark cycle, with food and water ad libitum.
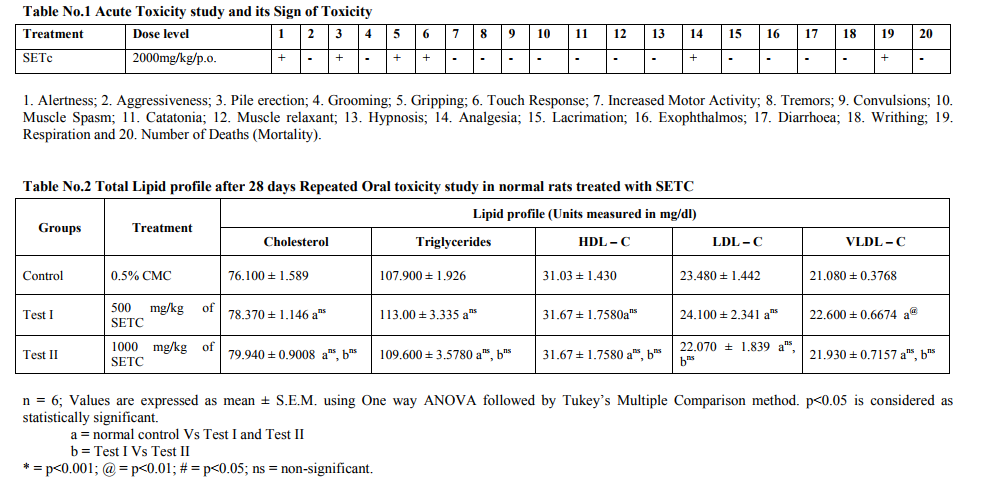
Toxicity studies Acute Oral Toxicity Study
Acute oral toxicity studies were performed following by OECD 423 Guidelines. Maximum dose of 2000mg/kg was selected and administered orally to a group of 3 animals in each step as shown in flow chart of Annex 2d [9]. Animals (n=6) were fasted for a period of 12 hrs and weighed just prior to drug administration. The test substance was administered in a single dose using a suitable intubation canula. After drug administration, food was withheld for a period of 3-4 hours. The animals were observed closely for 3 hrs and observation were continued for 24 hours. Any mortality or toxic signs produced were noted.
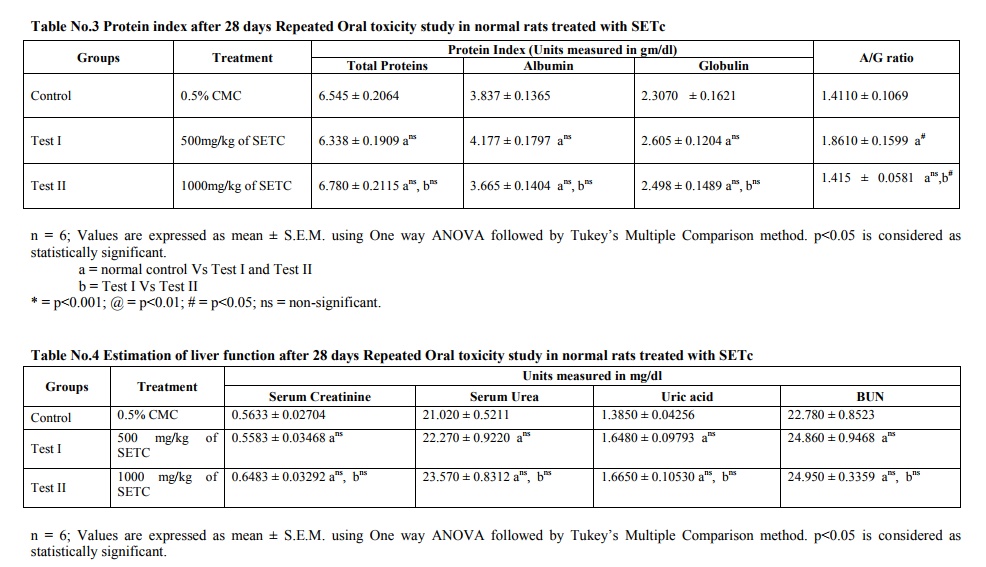
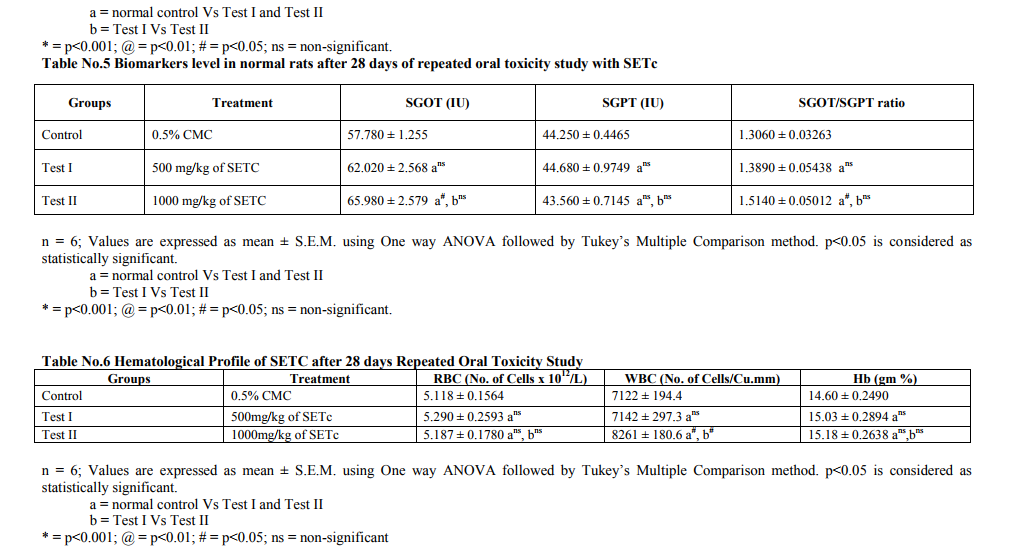
Repeated Oral Toxicity Study Repeated dose 28 day oral toxicity study was carried out according to OECD guidelines 407 [10]. Animals were divided into four groups of 6 each. Group I – received 0.5% CMC orally and served as vehicle control and groups II, III and IV – were received a daily dose of SETc 500, 1000 and 2000 mg/kg/p.o., respectively for a period of 28 days. Adjustments were made as necessary to maintain constant dose level in term of animal body weight. Animals were observed at least twice a week for 28 days, for any mortality and morbidity. The doses at which animals don‘t show any mortality or morbidity were chosen for the dose finding study. Animals that survived after 28 days treatment were euthanized with excess ether on 29th day and blood samples were collected through cardiac puncture for hematological and biochemical studies. Liver, kidney and Pancreas were dissected out for histopathological studies.
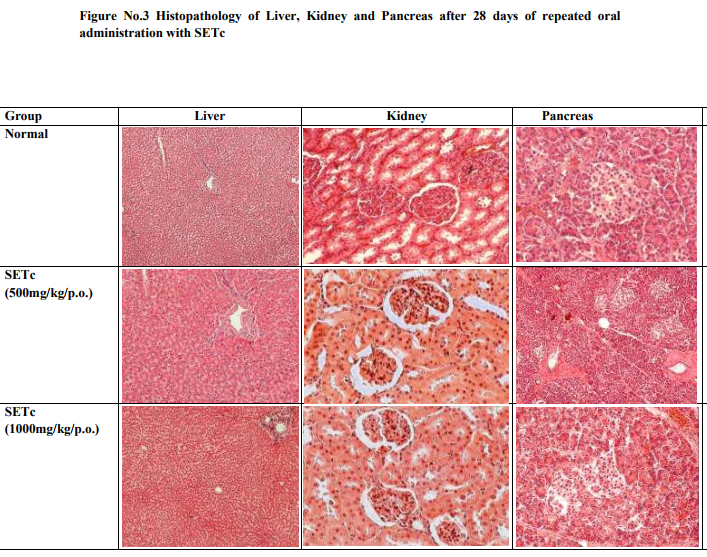
Histopathological Studies Various tissues like liver, kidney and pancreas were dissected out from each group of normal control and normal animals treated with SETc (500 and 1000 mg/kg/p.o., respectively). The collected tissues of respective groups were dipped in 10% formalin solution and stained with hemotoxylin and eosin for preparation of section by using of microtome. Histopathological observations were studied in Vaishnave Clinic, Chennai – 17. The histopathological studies carried out by using the method described by Kanai Mukherjee [11].
Effective dose finding-Experimental design Fasting of Animals and Induction of Experimental Diabetes
Animals were fasted for 16 hours before the induction of diabetes with Streptozotocin [2]. Animals made diabetic by an intraperitoneal injection of freshly prepared solution of STZ (45mg/ml in 0.01 m citrate buffer, pH 4.5). The diabetic state assessed in STZ - treated rats by measuring the non-fasting blood glucose concentration 48 hours post STZ injection using one touch glucometer. Only rats with blood glucose levels ≤ 200mg/dl were selected and used for experimental studies.
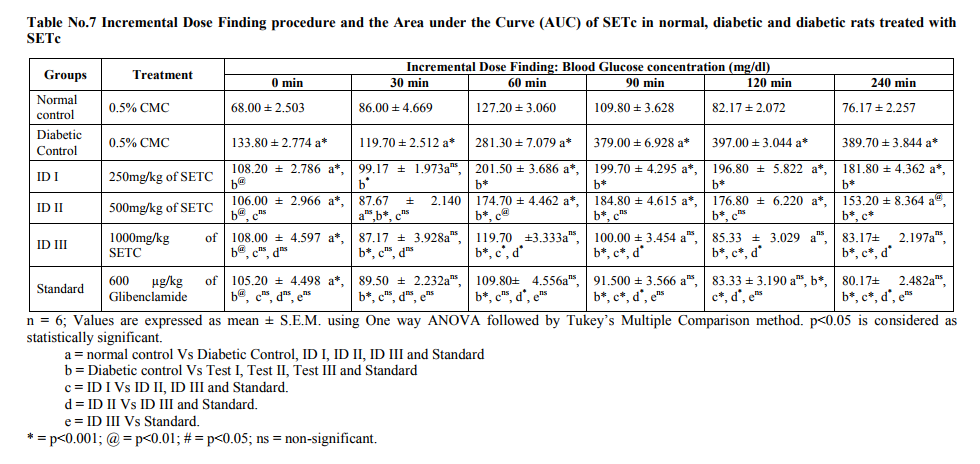
Incremental dose finding experiment: Modified method* With a slight modification of the method of Soon and Tan [2], the fasting glucose along with oral glucose loading after the administration of the incremental doses of the test drug were used. Animals were divided into 6 groups of 6 animals each. A normal and a diabetic control both receiving 0.5% of Carboxyl methyl cellulose suspension and test groups with diabetes receives Incremental Doses (ID) of ID I, II and III (250, 500 and 1000mg/kg/p.o., respectively). Finally a diabetic treated with Standard drug, Glibenclamide - 600µg/kg (as calculated from the human dose) kept studied for the comparison of the test drug treated groups. Blood glucose concentration was examined at a regular interval for a period of 4 hrs starting from 0 hr and at 1st, 2nd, 3rd, 4th hr after drug treatment using One Touch Glucometer. Concentration response curve and area under curve were studied.
Statistical analysisStatistical analyses were done using Graphpad prism software, Version 4. Dose response effect were studied using Curves and regression followed by Area under Curve (AUC) and other biochemical, hematological parameters were assessed through One way anova using Tukey‘s multiple comparison method, were values are expressed as mean ± SEM (n=6).
RESULTS
Toxicity Studies Prior to the clinical application of experimental data, it is pertinent to establish the safety of herbal preparation through toxicological assessments. In the current study therefore, the acute toxicity and the liver and kidney function parameters of animals treated with subchronic doses of the crude sedimental preparation of T.cordifolia were assessed. In addition the microanatomical changes, if any of the test drug in majors organs viz., liver, kidney and pancreas were also studied. Acute Toxicity study Acute toxicity study under OECD 423 guidelines a maximum tolerable dose of SETc (2000 mg/kg/p.o.,) was used to assess the mortality or morbidity rate and also toxic signs and symptoms of animals were studied. The result showed neither mortality nor signs of toxicity at this dose (2000mg/kg/p.o.,) as shown in table no.1. Repeated Oral Toxicity Study The maximum tolerable dose assessed from the acute toxicity study, i.e., 2000mg/kg/p.o., along with its 1/2 and 1/4 portion of the corresponding doses, 1000 and 500 mg/kg/p.o., respectively, were studied for 28 days repeated oral toxicity under OECD 407 guidelines. The mortality rate with 2000mg/kg found to show maximum number of deaths within 15 days from the start of the study. Animals treated with 500mg/kg and 1000mg/kg/p.o., of SETC respectively, didn‘t show any mortality or morbidity throughout the treatment period and there were no significant changes in the biochemical and hematological parameters when compared to control animals. The results are depicted in table nos.2 - 6. There was no significant change in the biochemical parameters including total triglycerides, cholesterol, HDL-C, LDL-C and VLDL-C in test animals treated with SETc (500 and 1000mg/kg/p.o.,) compared to control (Table No.2). There was no significant alteration in the serum protein level and the A: G ratio was found near to the normal control (p=ns) as shown in Table. 3. It was observed that the test drug I and II do not showed any alterations in the serum urea, uric acid, creatinine and BUN level as compared with that of the normal control (p=ns). Sub chronic treatment of SETc did not affect the AST and ALT levels in comparison to normal animals (p=ns) as shown (Table No.5). The RBCs and Hb contents of SETc treated rats were found to show no significant difference (p=ns) as compared with that of the normal group (Table No.6). The number of WBCs were found to show a slight increase in test group treated with 1000mg/kg/p.o., as compared with that of the normal (p<0.05).
Histopathology of organs after 28 days of repeated oral toxicity
The histopathological examinations of liver, kidney and pancreas of SETc treated animals showed normal architecture suggesting no detrimental changes and morphological disturbances in tissues treated with the test drug at the doses (500 and 1000mg/kg/p.o.,) for 28 days are depicted in figure no.3.
Effective dose finding Incremental dose finding experiment
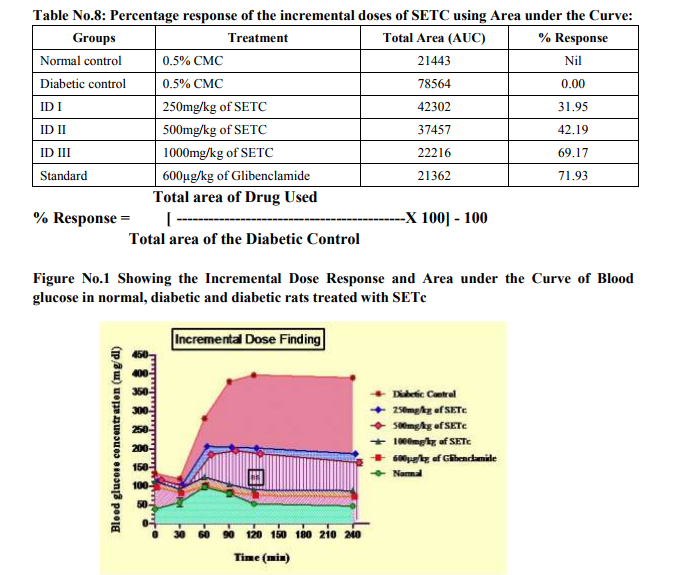
From the table no.7, it was observed that the ID‘s I, II and III (250, 500 and 1000 mg/kg/p.o., respectively) showed significant increase in BG after 30 min of GTT as compared with that of the normal (p<0.001). In addition, there was a significant decrease in the BG level of SETc treated diabetic animals when compared with that of the diabetic control (p<0.001). The ID III (1000 mg/kg/p.o.,) was alone found to maintain the BG level even after 90th, 120th, 240th min of glucose loading. It was also noted that test drug at the dose of 1000mg/kg/p.o., maintained the plateau range of BG level in GTT with that of the standard drug treated and normal control (Figure No.1).
Area under the Curve
As shown in the figure no.1, the filled area under the curve denotes the blood glucose concentration of the test drugs studied as their percentage response. It was observed from the table no.8; around 69.17 % response was produced at single dose of ID III (1000mg/kg/p.o.,) which was near to that of the standard drug used. Effective dose Figure No.2 shows the effective dose ranges of SETc where it reaches its therapeutic margin. The test drug ID I, II, III (200, 500 and 1000mg/kg/p.o., respectively) and standard drug (600 µg/kg/p.o, of glibenclamide) produced their onset of action after 30th min of glucose loading.
DISCUSSION
The toxicity profile of SETc at doses 500 and 1000mg/kg/p.o., after exposure to 28 days oral toxicity study, shown severe hepatic injury as a result of the metabolism of some of the toxic phytochemicals, found in the medicinal plants and failure of the elimination of those metabolized products by the liver are reported in the literature [12]. Albumin is the most abundant plasma proteins with the physiological role of maintenance of osmotic pressure, transportation of both exogenous and endogenous substances and serving as a protein reserve. The ability of the liver to synthesize albumin is diminished if the synthetic function of the organ is affected [13]. Increase plasma protein concentration may be due to dehydration and vice versa. From the result of the present study, serum protein profiles were not significantly different between the test group and control. This shows that synthetic function of liver of the animal exposed to subchronic doses of 500 and 1000mg/kg/p.o., of SETc is not affected. Additionally there was alteration neither in the globulin levels nor in the A: G ratio of the animals treated with sub-chronic doses of SETc. The total lipid profile of the test drug treated animals had no significant alterations in their levels which might be due to the activities of hepatic enzymes of test groups were not affected by treatment of SETc [14]. There was no change in the serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels which are useful indices for identifying inflammation and necrosis of the liver [15]. ALT measurements are more liver specific than the AST and its activity is usually greater than AST activity at early or acute hepatocellular disease [13]. AST on the other hand tend to be released more than the ALT in chronic liver diseases such as cirrhosis [13]. A marked elevation of ALT, however, in the presence of mild to moderate elevation of AST is suggestive of either hepatic toxicity or hepatic injury combined with other conditions [14]. Serum urea, uric acid, urea nitrogen and creatinine were examined as indicators for kidney function tests [16]. Hence there was no sign of alterations in the indicators level, it confirms that the kidney function of rats treated with SETc was not affected. No changes were observed in hematological profile and the slight increase in the WBC count was might be due to the immunomodulatory activity of T.cordifolia, as reported from the previous literature [17, 18 and19]. On surveillance of the hematological and biochemical parameters, the toxicity profile of 500 and 1000mg/kg/p.o., of SETc showed neither mortality nor alterations in the normal parametric results of rodents, which appeared to be failed at dose 2000mg/kg/p.o., (the acute dose of OECD 423; 2d which showed maximum number of deaths at the interval of 28 days repeated oral toxicity study) and thus, the effective dose for the primary study lies between these two doses a maximum tolerable dose and a mortality dose (1000 and 2000mg/kg/p.o., respectively) which were elucidated by applying this modified method of IDF procedure. Effective dose findings Even though, the onset of action of the incremental doses ID I, II and III have being started after 30th min of glucose load, the durability and maintenance of plateau of BGC was achieved only at ID III (1000mg/kg/p.o.). This might be due to satisfactory dose percent required for that response and its continued hypoglycemic effect (i.e. BG ≤ 100 mg/dl) even after 120th min evidences its effective onset and longer duration of action, which was not fulfilled by the prior low doses. Furthermore, the AUC clearly denotes % response for the drug used i.e. Blood glucose concentration is indirectly proportional to the percentage response. From the above dose–response studies, it was clear that the hypoglycemic activity of SETc was dose dependant.
CONCLUSION
The overall findings clearly establishes the fact, that the use of AUC with respect to BGC levels will be easier to predict the safer usage of oral chemical/herbal therapeutic preparations for the use of diabetes therapy with stabilized outcomes from preclinical datas. The statement is that, the use of this IDF procedure after toxicity studies performed with newer/forthcoming drugs for the antidiabetic therapy would be a great opportunity for the researcher to minimize the mortality rate of the animals used. And furthermore its glitters an awareness for using safer dose particular in long term therapy. This study could be serving as a preliminary evaluation for the identification of the safer therapeutic marginal dose with less risk of adverse effects. Further the levels of hypoglycemic action of this SETc have yet to be studied in future.
Finally, it was concluded that, the blood glucose concentration – a key factor for determining the AUC thereby fixing the effective concentration-response of the drug dose, using this modified method of incremental dose finding procedure.
ACKNOWLEDGEMENT Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are grateful to authors/editors/publishers of those articles, journals and books from where the literature for this article has been reviewed and discussed.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References:
1. Goodman and Gilman's. Pharmacokinetics and Pharmacodynamics. In: Laurence L. Brunton, Keith L. Parker, Donald K. Blumenthal, Iain L.O. Buxton, editors. Manual of Pharmacology and Therapeutics. 11th ed. New York: McGraw-Hill; 2008, pp.6-12.
2. Soon YY, Tan BKH. Evaluation of Hypoglycemic and Antioxidant activities of Morinda officinalis in STZ-induced diabetic rats. Singapore Med J 2002; 43(2): 077-085.
3. Singh SS, Pandey SC, Srivastava S, Gupta VS, Patro B, Ghosh AC. Chemistry and medicinal properties of Tinospora cordifolia (guduchi). Indian Journal of Pharmacology 2003; 35: pp.83-91.
4. Wadood N, Wadood A, Shah SAW. Effect of Tinospora cordifolia on blood glucose and total lipid levels of normal and alloxan-diabetic rabbits. Planta Med. 1992; 58: 131-136.
5. Prince PSM, Menon VP, Gunasekaran G. Hypolipidaemic action of Tinospora cordifolia roots in alloxan diabetic rats. Journal of Ethnopharmacology 1999; 64: 53-57.
6. Prince PSM, Menon VP. Short communication: Antioxidant action of Tinospora cordifolia roots in experimental diabetes. Journal of Ethnopharmacology 1999; 65: 277- 281.
7. Prince PSM, Menon VP. Hypoglycemic and other related actions of Tinospora cordifolia roots in alloxan-induced diabetic rats. Journal of Ethnopharmacology 2000; 70: 9-15.
8. Prince PSM, Menon VP. Antioxidant action of Tinospora cordifolia root extract in alloxan diabetic rats. Phytotherapy Research 2001; 15: 213- 218.
9. OECD/OCDE 423. Test procedure with a starting dose of 2000 mg/kg/bw. Annex 2d 2001; p.13.
10. OECD/OCDE 407. Repeated Dose 28-day Oral Toxicity Study in Rodents 1995; pp. 1-8.
11. Mukherjee KI. Medical Laboratory Technology. 1st Edition. New Delhi: Tata McGraw Hill Publications 1989: p.124.
12. Geidam MA, Pakman I, Laminu H. Effects of aqueous stem bark of Momordica balsamin. Linn on serum electrolytes and some haematological parameters in normal and alcohol fed rats. Pak. J. Biol. Sci. 2004; 7: pp.1430-1432.
13. Abdollahi M, Farzamfar B, Salari P, Khorram Khorshid HR, Larijani B, Farhadi M, et al. Evaluation of acute and sub-chronic toxicity of Semelil (ANGIPARS™), a new phytotherapeutic drug for wound healing in rodents. DARU 2008: 16(1); 7-14.
14. Tilkian SM, Conover MB and Tilkian AG. Clinical implications of laboratory tests. London. C.V. Mosby Company 1979: pp.3-44; 117-132; 154-159.
15. Whitby LG, Smith AF, Becket GJ. Lecture notes on Clinical chemistry. 4 th Ed. Oxford, London, Edinburgh, Boston, Melbourne; Blackwell Scientific Publications 1989; pp.38- 178.
16. Williams M.H. Nutrition for health, fitness and sport. Boston; McGraw-Hill 1999; pp.178-203.
17. Thatte UM, Dahanukar SA. Comparative study of immunomodulating activity of Indian Medicinal plants, lithium carbonate and glucan. Methods and findings in experimental and clinical pharmacology 1988; 10(10): 639-644.
18. Thatte UM, Dahanukar SA. Immunotherapeutic modification of experimental infection by Indian Medicinal Plants. Phytotherapy Research 1989; 3: 43-49.
19. Mathew S, Kuttan G. Antioxidant activity of Tinospora cordifolia and its usefulness in the amelioration of cyclophosphamide induced toxicity. Journal of Experimental and Clinical Cancer Research 1997; 16(4): 407- 411.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License