IJCRR - 4(10), May, 2012
Pages: 19-26
Date of Publication: 25-May-2012
Print Article
Download XML Download PDF
ISOLATION AND CHARACTERIZATION OF POLYGALACTURONASE PRODUCING BACTERIA FROM SOIL AND VEGETABLE WASTE
Author: Amit Pratush, Anupama Gupta, Gitanjali Vyas
Category: General Sciences
Abstract:Fifteen different bacterial strains were isolated from the vegetable wastes, were screened for the production of polygalacturonase enzyme. The most responding strains which exhibited maximum Polygalacturonase activity is named as PG-2 isolate. This isolate is gram negative, rod shaped and nonsporulating in nature. The cultivation of selected strain (PG-2 isolate) in liquid medium resulted in high quality of polygalacturonase enzyme. The maximum Polygalacturonase activity was reached in 72h of growth in medium having citrus pectin as carbon source and mixture of casin hydrolysate + yeast extract (1% v/v) as nitrogen source. This organism exhibited maximal Polygalacturonase activity at pH 6.5 at incubation temperature of 30oC. The PG-2 isolate exhibited maximum Polygalacturonase activity in sodium citrate buffer pH 5, when incubated at 40oC for 15 min.
Keywords: Gram negative, Polygalacturonase enzyme, PG-2 isolates, Casin hydrlyaste, Screening
Full Text:
INTRODUCTION
Pectinases are amongst the most important industrial enzymes widely distributed in bacteria, fungi and plants [1, 2, 3, 4]. They are of great significance with tremendous potential to offer to industry [5]. Their commercial application was first observed in 1930s for the preparation of wines and fruit juices. They are one of MATERIAL AND METHODS the upcoming enzymes of the commercial sector, especially the juice and food industry [6, 7]. Pectin is the complex, colloidal acidic polysaccharide present primarily in the cell wall and middle lamellae of fruits and vegetables and is one of the most widely available polysaccharide in nature next to cellulose, starch and chitin. Hydrolysis of pectin is obtained by the synergistic action of a pectinolytic system which hydrolyzes pectic substances. The most extensively studied pectinolytic enzymes are: Protopectinases, Polygalacturonases, Lyases, Pectinesterases. Protopectinases enzyme catalyze the solubilization of protopectin. Polygalacturonases are the most extensively studied among the family of pectinolytic enzyme. Amino acid residues participating in polygalacturonase-inhibiting protein (PGIP) binding to homogalacturonan in the cell wall have been determined [8]. The enzyme is composed of a backbone of D-galacturonic acid which may be methylated as it may be substituted. Polygalacturonases show extensive variation in physical and chemical properties [9].
Pectinases have been used to increase the yield and to clarify juices [5, 6, 10]. This particular application of pectinases can be of tremendous importance to Himachal Pradesh as it deals with large amount of horticultural produce especially apple. The apple juice faces the problems of turbidity and suspended particles like pectin, cellulose and hemicelluloses. Due to these factors, the juice fails to match international standards. The quality of juice can be improved by appropriate treatment with pectinolytic enzymes leading to the increased market potential, thereby, providing a significant boast to the economy of hill state. Pectinases have a lot to offer to biotechnology industry.
MATERIAL AND METHODS
Sampling and Enrichment Twenty samples were collected from the soils and from some rotten vegetables collected from the shops of Solan area. Polygalacturonase producing microorganisms were isolated using the enrichment process by Layh et al., [11], with an additional culturing on nutrient agar prior to the transfer to defined medium. After appropriate dilution samples were plated on nutrient agar and incubated at 30 ºC for 36 h. Different colonies were randomly picked up, numbered and maintained on nutrient agar slopes.
Screening and selection of Polygalacturonase producing strains
Isolated pure cultures were screened for the Polygalacturonase enzyme production by using the standard assay method of Nelson [12] and Somogyi [13]. The clonies showing highest Polygalacturonase activity were selected for further optimization and was named as PG-2 isolate.
Enzymatic assay
The polygalacturonase activity was measured by different methods. Firstly, the activity was detected on solid medium by using ruthenium red dye [14]. Secondly, Polygalacturonase activity was determined by measuring the increase in production of reducing ends, with polygalacturonic acid as substrate. Polygalacturonase activity was assayed by quantifying reducing groups expressed as galacturonic acid units which had been librated during the incubation of 200 μl of 1% (w/v) polygalacturonic acid in 10 mM sodium acetate buffer, pH 5.1 (optimum pH) with 200 μl of suitably diluted enzyme at 25 ºC, for 5 min by DNS (dinitrosalicylic acid) method defined by Miller, [15]. One unit of polygalacturonase was defined as the amount of enzyme required to release 1 μmol of galacturonic acid (as a standard) from the polygalacturonic acid/ml/min.
Morphological and physiological characters of PG-2 isolate
Gram character and morphology of the PG-2 isolate was determined. Catalase activity of the microorganism was checked by diluted solution of hydrogen peroxide. Optimization of production conditions of Polygalacturonase enzyme from selected PG2 isolate Using one parameter variant at a time (OVAT) approach, the culture conditions for the production of Polygalacturonase enzyme by selected PG-2 isolate were optimized. The optimization of different production conditions was done in triplicates.
Media composition
Several different media were prepared during this study. Sterilization was carried out at 121 °C and 15 psi for 20 min. The initial pH in all cases was 5.6. An appropriate defined medium was chosen to compare the results, in terms of enzyme production. All cultures were incubated at 30°C in an orbital shaker at 160 rpm and samples were collected every 24 h. The experiments were carried on in 250 ml conical flasks with 50 ml of medium. Then samples were filtered through Whatman No.4 filter paper, and the filtrate was evaluated for enzyme activity. Media pH The selected PG-2 isolate was cultured in the medium with pH varying from 5.0–8.0 and the Polygalacturonase activity was determined. Incubation temperature and incubation time optimization The effect of incubation temperature and time on the production of Polygalacturonase enzyme by PG-2 ioslate was studied. This microorganism was grown at various temperatures, i.e. 25-50 ºC for 24 to 120 h.
Effect of Carbon and Nitrogen sources and their concentrations
Various Carbon sources [Polygalacturonic acid, citrus pectin, glucose, fructose, galactose and maltose] and nitrogen sources [NaNO3, urea, casein hydrolysate and yeast extract] were used in the production medium at a concentration of 1% w/v to check their effect on Polygalacturonase production. The concentration of these two sources (i.e. carbon and nitrogen) was also optimizing to determine the optimal concentration needed for the maximal production of Polygalacturonase in medium. The media containing citrus pectin as carbon source and casein hydrolysate and yeast extract was used as control. Optimization of Reaction conditions of Polygalacturonase enzyme from selected PG2 isolate Reaction conditions for assay of Polygalacturonase activity in crude extract of PG-2 isolate were optimized by using various buffer systems [phosphate buffer, sodium acetate buffer, citrate buffer and tris Hcl buffer], buffer pH (3.5-6.0), temperature (30-50oC), reaction time (5-25 min) and effect of metal ions on crude enzyme.
RESULT AND DISCUSSION
Isolates Fifteen colonies were selected from various samples as potential polygalacturonase producers, among them Gram negative rod named PG-2 was found to be the best enzyme producer and hence was used for further optimization of parameters and conditions for enhanced production of enzyme.
Optimization of production conditions of Polygalacturonase enzyme from selected PG2 isolate
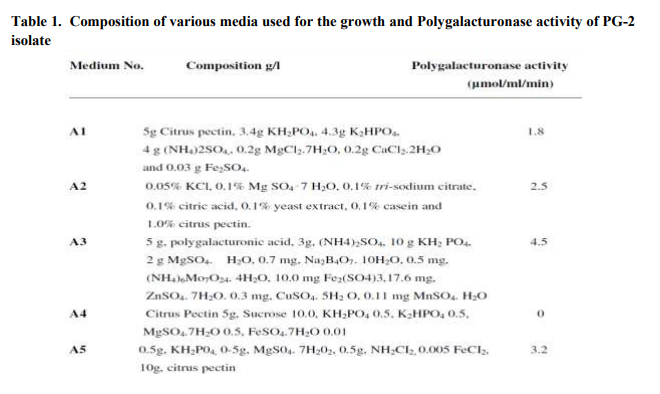
The PG-2 isolate exhibited maximum growth in A3 media. The composition of different media (A1-A5) is given in Table 1. The Polygalacturonase activity was 2.5, 1.8 and 1.4 times higher in A3 medium as compared to A1, A2 and A5 medium, respectively, whereas the PG-2 isolate, did not show any growth or activity in A4 medium (Table 1). The other standard strains Trichoderma koningii Oud, isolate CF-1 was grown in A5 medium and Aspergillus awamori exhibited growth and Polygalacturonase activity in A1 medium [16, 17]. Medium pH exerted significant effect on growth and Polygalacturonase activity as the maximum growth and enzymatic activity (5.2µmol/ml/min) was recorded at slightly acidic pH i.e. 6.5 (Fig.1a). At pH 5.0 and 7.5 there is a loss of 61.5 and 51.9 %, Polygalacturonase activity, respectively. The other Polygalacturonase producing organisms such as Bacillus sphaericus (MTCC 7542), Tetracoccosporium sp. and Trichoderma koningii Oud, isolate CF-1 exhibited optimum Polygalacturonase activity at pH 6.8, 5 and 5.6, respectively [16, 18, 19]. The incubation temperature and time also had significant effect on Polygalacturonase activity of PG-2 isolate. The isolated organism exhibited optimum Polygalacturonase activity (6.2µmol/ml/min) in A3 medium pH 6.5 at 30oC (Fig.1b), after the incubation of 72 h at same temperature the isolated organism exhibited optimum activity of 6.4 µmol/ml/min. (Fig.1c). Whereas the other Polygalacturonase producing organisms such as Bacillus sphaericus (MTCC 7542), Tetracoccosporium sp. and Aspergillus awamori exhibited maximum Polygalacturonase activity at 30-35oC after 72, 48 and 96 h of incubation, respectively [17, 18, 19]. Medium supplemented with citrus pectin 1% (w/v) exhibited maximal Polygalacturonase activity (6.9 µmol/ml/min) (Fig. 1d). The other carbon sources such as glucose, fructose, galactose and maltose inhibit the Polygalacturonase production and a drastic decrease (up to 70%) in enzyme production was recorded. Glucose and fructose inhibited polygalacturonase biosynthesis, and synthesis of the enzyme in relatively small amounts with low enzymatic activity, this fall in Polygalacturonase activity may be due to catabolite repression [18, 19]. The optimization of best nitrogen source was carried out by supplementing the media with different nitrogen sources such as NaNO3, urea, casein hydrolysate and yeast extract. The PG-2 isolate exhibited maximum Polygalacturonase activity (7.0 µmol/ml/min) in the presence of casein hydrolysate (CH) and yeast extract (YE) 1% (w/v) (Fig. 1e). Jayani et al., [18] and Kashyap et al., [20] also used the same nitrogen sources for the Polygalacturonase production from Aspergillus awamori and Bacillus sp. DT7. The concentration of carbon and nitrogen source varies from 0.5% to 2.5% and PG-2 isolate exhibited maximal Polygalacturonase activity at concentration of 1% w/v of both carbon source (citurs pectin) and nitrogen source (Casein hydrolysate and Yeast extract) (Table 2).
Optimization of Reaction conditions of Polygalacturonase enzyme from selected PG2 isolate
The PG-2 isolate exhibited maximum Polygalacturonase activity (7.5 µmol/ml/min) in 0.1 M sodium acetate buffer having pH 5 (Fig.1 f and g). Saeed et al., [19] also describe the optimum Polygalacturonase activity by Tetracoccosporium sp. in the same buffer i.e. sodium acetate buffer pH 5 having 10 mM ionic strength. Whereas the other Polygalacturonase producing strains such as Trichoderma koningii showed the maximum activity in sodium polypectate in McIlvaine's buffer (35 mMphosphate) pH 5.0 [21]. The optimum pH for Aspergillus niger CH4 Polygalacturonase has been reported to be in range of 4.5-6.0 [22]. Polygalacturonase from Rhizoctonia solani [23], Thermoascus aurantiacus [24], P. expansum and A. alliaceus [25] exhibited maximal hydrolytic activity at pH 4.8, 5.0 and 5.5, respectively. The optimum reaction temperature for Polygalacturonase of PG-2 isolate was 40oC (Fig.1 h). Several Polygalacturonase producing organisms have different temperature profiles. For example the optimum temperature activity of S. sclerotiorum is 45 ºC and polygalacturonase activity is mostly inactive at 65 ºC [26]. In A. kawachii polygalacturonase did not show any activity at pH 5.0 [27]. Polygalacturonase from S. chevalieri and C. albidus, were found to have optimum temperature of 25 and 37 ºC, respectively [28]. T. aurantiacus and A. alliaceus exhibit maximal polygalacturonase activity at optimum temperature of 65 and 35ºC, respectively [24, 25]. The PG-2 isolate exhibited maximum Polygalacturonase activity at incubation time of 20 min (Fig.1 i) as incubation time rises the enzymatic activity starts decreasing. The Aspergillus awamori exhibited maximum Polygalacturonase activity at the reaction time of 15 min. [18]. The polygalacturonase activity was measured at pH 5.0 in the presence of various metal ions (1 mM). Metal ions such as Mn2+ and Ag3+ increased polygalacturonase activity by almost 50 % (Table 3). Iodoacetamide and idoacetic acid did not inhibit the enzyme activity at 1 mM concentration which indicated that cysteine residues are not the part of catalytic site of polygalacturonase [19]. It is also interesting to note that all the surface-active detergents i.e. tweens (20 and 80), triton X-100 and SDS, affects the polygalacturonase activity by up to 35 %. Perhaps this is due to the fact that the surface-active reagents might have increased the turnover number of polygalacturonase by increasing the contact frequency between the active site of the enzyme and the substrate by lowering the surface tension of the aqueous medium [1]. Ca2+, Zn2+,Cu2+, Co2+, Ni2+, Ba2+ , Fe3+, Al3+, Cr+3, K+ and EDTA partially inhibited the Polygalacturonase activity (Table 3). CONCLUSION A new polygalacturonase producing bacterial strain i.e. PG-2 isolate was isolated from the vegetable waste and the production and reaction conditions of this strain were optimized by using OVAT technique. This organism produces maximal polygalacturonase after 72 h of incubation in the presence of citrus pectin as a carbon source and mixture of casin hydrolysate and yeast extract (1% v/v) as nitrogen source. The crude Polygalacturonase is stable in sodium citrate buffer pH 5 after the incubation of 15 min at 40oC. ACKNOWLEDGEMENT Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Kapoor M et al. Process Biochemistry 2000. 36. 467-473p.
2. Kashyap D et al. World Journal of Microbiology and Biotechnology 2000. 16. 277-282p.
3. Souza, JVB et al. Process Biochemistry 2003. 39 (4). 455–458p.
4. Xavier SS et al. Folia Microbiologica 2004. 49. 46-52p.
5. Jayani RS et al. Process Biochemistry 2005. 40. 2931-2944p.
6. Kashyap DR et al. Bioresource Technology 2001. 77. 215-227p.
7. Sharma A et al. World Journal of Microbiology and Biotechnology 2007. 23 (4). 483-490p.
8. Protsenko MA et al. Biochemistry 2008. 73. 1053-62p.
9. Martins ES et al. Antonie Van Leeuwenhoek 2007. 91. 291-299p.
10. Solehah A et al. Journal of Food Science and Technology 1994. 31. 508-510p.
11. Layh N et al. Applied Microbiology and Biotechnology 1997. 47. 668–674p.
12. Nelson N. Journal of Biological Chemistry 1944. 153. 375–380p.
13. Somogyi M. Journal of Biological Chemistry 1952. 95 (1). 19–23p.
14. Gainvors A et al. FEMS Microbiology Letters 2000. 183. 131-135p.
15. Miller GL et al. Annals of Chemistry 1959. 31. 426-427p.
16. Fanelli C et al. Journal of Genetics and Microbiology 1978. 104. 305-309p.
17. Abbasi H et al. Iranian Journal of Biotechnology 2011. 9 (1). 50-55p.
18. Jayani RS et al. Enzyme Research 2010. 1- 5p.
19. Saeed A et al. Iranian Journal Chemical Engineering 2007. 26 (1). 1-8p.
20. Kashyap DR et al. Bioresource Technology 2003. 88 (3). 251–254p.
21. Fanelli C et al. Transactions of the British Mycological Society 1977. 68. 291-294p.
22. Acuna-Arguelles ME et al. Applied Microbiology and Biotechnology 1995. 43 (5). 808–814p.
23. Marcus L et al. Physiology and Molecular Plant Pathology 1986. 29. 325-336p.
24. Martins ES et al. Process Biochemistry 2002. 37. 949-954p.
25. Shubakov AA et al. Chemical Technology 2002. 7. 65 -68p.
26. Riou C et al. Applied Environmental Microbiology 1992. 58. 578-583p.
27. Contreas Esquivel JC and Voget CE. Journal of Biotechnology 2004. 110. 21- 28p.
28. Blanco P et al. FEMS Microbiology Letters 1999. 175. 1-9p.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License