IJCRR - 4(12), June, 2012
Pages: 06-19
Print Article
Download XML Download PDF
QUANTIFICATION OF SUBGINGIVAL MICROFLORA IN A PERI IMPLANTITIS PATIENT USING REAL TIME POLYMERASE CHAIN REACTION TECHNIQUE - A PILOT STUDY
Author: PSG Prakash, D.J. Victor, S. Vaishnavi, Ponsekar A. Abraham
Category: Healthcare
Abstract:Aim and Objective: Peri-implantitis is a chronic progressive marginal infection which is defined as an inflammatory reaction affecting the tissues surrounding osseointegrated dental implants resulting in loss of supporting bone. The microflora of patients with implants who are edentulous mainly consists of gram positive facultative cocci and non motile rods. Predominantly Streptococcus sanguis, Streptococcus mitis are found in a healthy, stable implant whereas motile rods, Spirochetes, Fusiforms and filaments are infrequently found. Microbiota around the failing implant in edentulous patients usually consists of gram negative anaerobic rods. In partially edentulous patients, peri implant microorganisms in a stable implant majorly consists of motile rods, Spirochetes and cocci. In a failing implant in partially edentulous patients high proportions of P.micros, P.intermedia, C.rectus and Fusobacterium species were observed (Alcoforado et al in 1994). The concept that the composition of subgingival microflora around implants in partially edentulous patients is said to be resultant of composition of flora around the teeth. Based on this principle we set out to identify the presence of putative periodontal pathogens on teeth in a predominantly edentulous arch with a metallic coping with periodontitis and a site in the same mouth with peri implantitis. Materials and Method: Subgingival plaque samples were collected from a partially edentulous patient using a paper point from the periimplantitis lesion with a probing depth of 8-9mm and
a tooth with deepest pocket with a probing depth of 6mm and we analyzed the five putative periodontal pathogens namely, P.gingivalis, P.intermedia, P.nigrecens, T.denticola and T.forcythia using Real Time Polymerised Chain Reaction (RTPCR) technique. Results: P.Gingivalis and P.Intermedia , P.Nigrecens had a 3 fold increase and T.Denticola had 1 fold increase when compared to the periodontitis ( teeth) site, and T.Forsythia was found in trace amounts at the periimplantitis site and was completely absent at the periodontitis( teeth) site.
Keywords: Implants, Periimplantitis, Periimplant microflora, Ball abutments, Over denture.
Full Text:
INTRODUCTION
From the past to present, the various treatment modalities in Dentistry for the replacement of missing teeth starting from Removable partial denture to Fixed partial denture, Dental Implants play a major role in the present scenario. Osseointegrated Titanium implants have become important alternative to conventional prosthesis for replacement of missing teeth1,2 . With increasing demand for dental implants,dental implant failure is also being reported more frequently3-8 . Hence the appropriate management of dental implants is always a quest to accomplish fruitful results. But, the Implant Dentistry often fails to impart us knowledge about the significance of regular assessment and the monitoring of the peri implant conditions. This often leads to peri implant infections and peri implant failures. Implant failures can be categorized as early and late. Early failures occur before osseointegration and prosthetic rehabilitation has taken place and late failures occurr afterwards9 . Late failures maybe sub classified into late-early and late-delayed depending on whether they occur during or after the first year of loading. Late-delayed failures are likely due to changes in the loading conditions in relation to quality or volume of bone and peri implantitis10. Aleast 10% of implant failures have been reported due to peri implantitis11 . The peri implant tissues of dental implants are colonized by a large variety of oral microbial complexes. The microflora that is present in the oral cavity before implant placement determines the composition of the newly establishing microflora around implants12 . Peri implant infections are peri implant mucositis which progresses onto peri implantitis, depending on the severity of the infection. Peri implant mucositis is defined as a reversible inflammatory reaction in the soft tissues surrounding an implant13 . Peri implantitis may be defined as the inflammatory process affecting the tissues around an osseointegrated implant in function, resulting in loss of supporting bone14. Apart from the microbial shift; local circumstances ( i.e. unsatisfactory oral hygiene, bone defects, deep pockets, overload ) as well as systemic conditions ( i.e. diabetes, smoking, genetic factors) may be important contributing factors as well15,16,17 On a clinically stable implant, S.sanguis and S.mitis are the most predominant organisms; while motile rods, spirochetes, fusiforms, and filaments are infrequently found18. When an implant failure occurs; a paradigm shift takes place in the microflora in which gram positive organisms basically become gram negative. Wherein the cocci becomes rods; immobile facultative anerobes become motile and strict anerobes19. According to Klinge et al in 2005, the medium of transfer of infection in oral cavity is saliva which proves that the transfer of periodontopathogenic bacteria from the natural teeth site to the vicinity of implants is saliva20 . In another study, Mombelli and associates21 isolated an increased proportion of gram negative anaerobic rods in edentulous and partially edentulous patients, especially P intermedia, Fusobacteria and Spirochetes. Quirynen and co workers22 in their study isolated the periodontal pathogens P.intermedia, P.nigrescens, A.actinomycetumcomitans and P.gingivalis of their samples of partially edentulous patients and none in edentulous patients. There is limited information in the literature regarding the incidence of peri implant disease and presence putative periodontal pathogens in a partially edentulous patient having a metal coping over three teeth in one quadrant with an implant in the adjacent quadrant supporting an overdenture against a complete denture in the opposite arch. Hence the present study aims to identify and quantify putative periodontal pathogens at peri implantitis site and to compare it with isolated teeth sites with metal copings and to discuss the differences that might be present.
MATERIALS AND METHODS
Patient Information
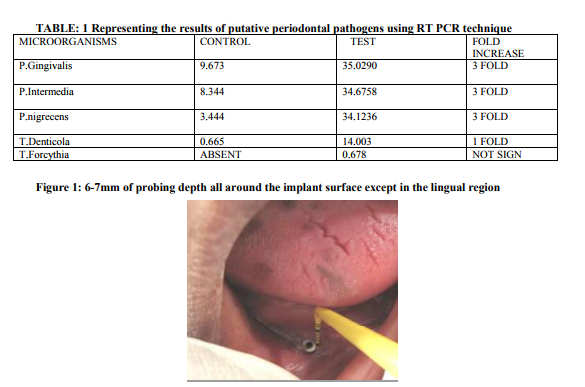
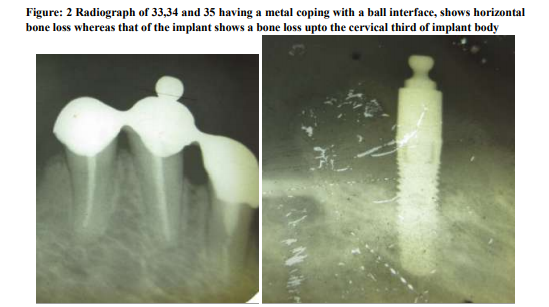
A 53 years old female , was referred from the department of Prosthodontics to the department of Periodontology and Oral Implantology, SRM Dental College, Bharathi salai, Ramapuram, Tamil Nadu, Chennai, India; for the management of a failed dental implant in the 44 region, that was placed 3 years back. In the lower arch, the patient was partially edentulous with three teeth bearing a metal coping with a ball interface and on the adjacent quadrant she had a single implant with a ball abutment, both supporting an overdenture. She was given a complete denture in the maxillary arch opposing the lower overdenture. The implant was placed in the year 2008 and the patient reported back to the department after 3 years with a complaint of pain in relation to the lower implant region for past two weeks. In the deparment of Peridontics it was diagnosed as a Late-delayed implant failure. On Clinical examination revealed a probing depth of 6-7mm all around the peri implantitis site except in the lingual region (fig 2). The remaining teeth in the opposite quadrant showed a probing depth of 4-5mm. On the otherhand, the radiographical examination revealed horizontal bone loss in relation to the remaining three teeth and there was a bone loss upto the cervical third of the implant body at the 44 region (fig 3). Probing pocket depth has been found to be the most important clinical parameter in relation to the peri implant microbiota23. With increasing pocket depth, other morphotypes ( motiles and spirochetes ), as well as for the total number of organisms were observed23 . The ethical clearance for this study was obtained from the ethical committee board of SRM University, Bharati Salai, Ramapuram, Chennai- 89. The patient who participated in the study was detailed about the study protocol and her informed consent was obtained. The authors report no conflict of interest related to the study.
Procedure for Plaque Sample Collection
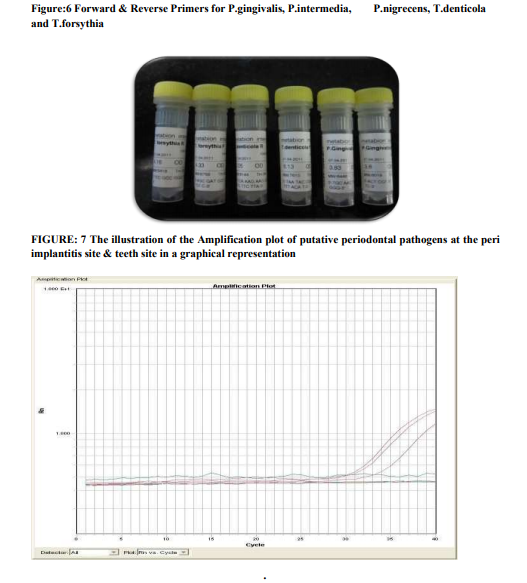
Subgingival plaque samples were collected from the mesial and buccal sites of teeth and peri implantitis site with the deepest pocket by means of a sterile paper points (# 35, US Patent no - 5,833,458) (fig 4and5). Samples were placed in 0.1 mL Ethanol (99.9% pure, M.W. 46.08). After all the samples were collected, the samples were then taken to the central research laboratory, Sri Ramachandra Medical College, where the samples were then analysed for quantification of periodontal pathogens by using the REAL TIME PCR (fig 6). The processing reagent (fig 7), PCR reagents and Master Mix Kit (fig 8) were obtained from Applied Biosystems, Warrington, UK.
PRINCIPLES OF THE PCR
The Polymerase Chain Reaction (PCR) is a technique in molecular biology to amplify a single or few copies of a piece of DNA of Red complex bacteria across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence.
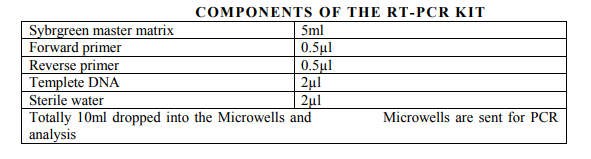
PRIMER DESIGN AND SELECTION
Species-specific primers (Inqaba Biotech Industries Ltd) were used to detect the presence of P.gingivalis, P. intermedia, P.nigrscens, Tforcythia, T.denticola (fig 9). The expected product lengths were 641 bp for T. forsythia, 404 bp for P. gingivalis and 316 bp for T. denticola. A pair of ubiquitous primers product length (602 bp) which matched most bacterial 16S rRNA genes at the same position was used as a positive control for the PCR reaction.
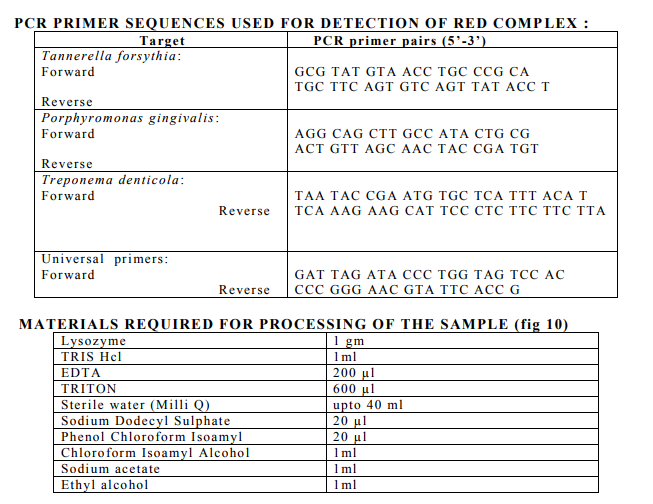
PROCESSING OF SAMPLES- (m-RNA ISOLATION)
Collected plaque samples were stored in Eppendorf tubes containing Ethanol solution (99%) at -80? C. The samples were then centrifuged under 7000 rpm for 5minutes (fig 11). The supernatant was removed and discarded. Mixture of 1gm of lysozyme powder was mixed with TRIS Hcl (1ml), EDTA 200 µl, TRITON 600 µl and sterile water Milli Q (making upto 40ml), then vortexed for 5 min and 0.8 µl of all the mix was added to each Eppendorf sample and incubated for 30 minutes at 37C (fig 12) .Sodium Dodecyl Sulphate 20µl was added to the sample , vortexed again and incubated for 30 minutes at 37C. To this centrifuged under 10000 rpm for 10 minutes and the supernatant was extracted and added to a new Eppendorf tube.
Choloroform Isoamyl Alcohol 1 ml added to the new tubes and centrifuged under 10000 rpm for 10 minutes and the supernatant was discarded and added to new Eppendorf tube. To this Sodium Acetate 1ml and Ethyl Alcohol 1ml was added and centrifuged under 10000 rpm for 10 minutes and the supernatant was discarded and Ethanol 500 µl was added to the remaining of the sample and centrifuged 10000 rpm for 5 min. The supernatant was discarded and the remaining pellet was dried for 2 hrs and add 30 µl sterile water was added to it,then freezed and sent for PCR analysis.
Quantification of Porphyromonas Gingivalis, Porphyromonas Intermedia, Porphyromonas Nigrecens, Tannerella Forsythia, Treponema Denticola using PCR Analysis
To setup PCR reaction, commercially available Sybrgreen master mix - 5ml was added with another 5ml containing Forward primer-0.5µl, Reverse primer -0.5µl, Template DNA -2µl, and Sterile water -2µl. The total 10 ml of the mix was dropped into the microwells. Then the microwells were kept for PCR analysis in the PCR machine (fig 13).
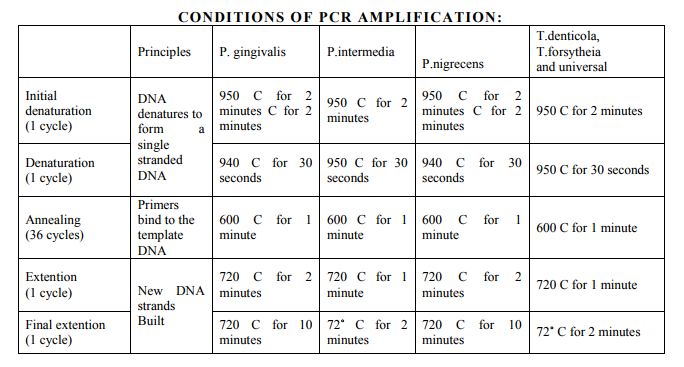
Evaluation of RT-PCR amplification
Real-time PCR data are quantified absolutely and relatively. This study employs relative quantification which relies on the comparison between expression of a target gene versus a reference gene and the expression of same gene in target sample versus reference samples. Ct (Cycle threshold) –values of each individual in each group with their pro-inflammatory mediator and their subsequent endogenous control values (standard value) obtained form RT-PCR analysis. PCR amplification efficiency can be either defined as percentage or as time of PCR product increase per cycle (from 1 to 2). . The efficiency-calibrated model is a more generalized Ct model. Ct number is first plotted against cDNA input (or logarithm cDNA input), and the slope of the plot is calculated to determine the amplification efficiency (E). Ct for each gene (target or reference) is then calculated by subtracting the Ct number of target sample from that of control sample. As shown in Equation 1, the ratio of target gene expression in treatment versus control can be derived from the ratio between target gene efficiency (Etarget) to the power of target Ct (Cttarget) and reference gene efficiency (Ereference) to the power of reference Ct (Ctreference).
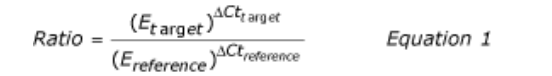
Amplification is done by the values obtained from ct. Lower the value, higher the expression of the microorganisms.
STATISTICS
Since we have dealt with only one sample and this being a pilot study, we have done a descriptive analysis.
RESULTS
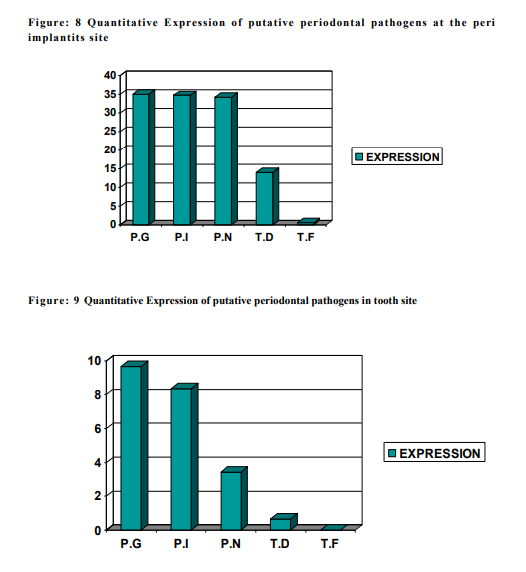
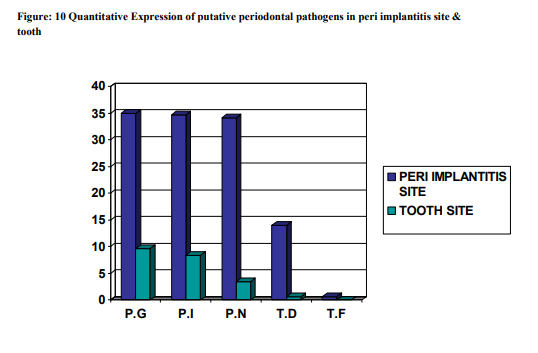
The subgingival plaque samples collected from the two sites namely the peri implantitis site ( i.e.Test ) and the Teeth site ( i.e.Control ) were analyzed using the RT-PCR technique. Using the RT-PCR method,the organisms studied are P.gingivalis, P.intermedia, P.nigrscens, T.denticola and T.forcythia. According to the results obtained at the end of the study (table 1), P.G ranked the highest with a score of 35.0290 at the peri implantitis site and 9.673 at the teeth site. P.G was followed by P.I, P.N, T.D and whereas T.F was the least with the score of 0.678 at the peri implantitis site and completely absent at the teeth site. The Amplification plot of putative periodontal pathogens at the peri implantitis site and teeth site in a graphical representation, was plotted against Threshold Cycle and Temperature (fig 14). It demonstates an elevated graph level of P.G, P.I, P.N, T.D and T.F respectively at the peri implantitis site whereas at the teeth site these bacterial species demonstrates almost a flat graph level. A bar graph representing the Quantitative Expression of putative periodontal pathogens at the peri implantits site (fig 15), depicts the variation in the range of the bacterial species. According to this, P.gingivalis was the highest with a score of 35.0290, P.intermedia scored 34.6758 followed by P.nigrecens with a score of 34.1236, T.denticola was minimum with 14.003 and the least was T.forcythia with a score of 0.678. Another Bar graph (fig 16) was drawn to demonstrate the Quantitative Expression of putative periodontal pathogens at the teeth site. Even here P.G was the highest with a score of 9.673, follwed by P.I which was 8.344, P.N scored 3.444, T.D was 0.665 and T.F was completely absent. A comparative Bar graph was drawn (fig 17) depicting the Quantitative Expression of putative periodontal pathogens at the peri implantits site and teeth site. According to this chart, P.G and P.I,P.N showed a three fold increase at the peri implantitis site than at the teeth site. Whereas T.D showed a one fold increase at the peri implantitis site compared to the teeth site. T.F was present only in trace amount at the peri implantitis, which was totally absent at the teeth site.
DISCUSSION
Implant failures are classified as Early or Late. Late failures maybe further subclassified into Late- Early or Late-Delayed. 80% of failures were attributed due to Late-delayed failures. Even in our study,it was a case of Late-Delayed failure of the implant which led to peri implantitis. The transmucosal abutment of the osseointegrated dental implant serves as a surface for bacterial colonization of the microbial biofilms. Like gingival crevice around the natural tooth; the peri implant mucosa which covers the alveolar bone, is closely adapted to the implant. Microbial colonization and the ensuing inflammatory reactions in the peri implant tissues might be the analogous to key events in the pathogenesis of periodontitis24,25. It is believed that the source of infecting bacteria is mainly plaque from residual teeth or saliva, and that microbiota around the implants tends to be similar to that of residual teeth. The periodontal status of remaining teeth would thus determine the bacterial composition of peri implantitis site26. Our study using RT-PCR technique quantifies the presence of P.gingivalis, P.intermedia, P.nigrcens, T.denticola and T.forcythus at the peri implantitis site and tooth site in which the peri implantitis site showed a 3 fold increase of P.G,P.I and P.N and a 1 fold increase of T.D when compared to that of the teeth with metal coping. Our study was in concurrence with the previous studies by Quirynen et al (1999), Mombelli et al (1990,2001) and Papaioannou et al (2007) were they observed a greater amounts of P.gingivalis, P.intermedia, P.nigrcens ,T.denticola and T.forcythia. In our study we saw for all the above microbes except for T.forcythia. It could be plausibly substantiated by the difference in the attachment of the biofilm on the metal coping with a ball abutment on teeth to support the overdenture than on a normal tooth without a metal coping, and biofilm on the rough surface of implant. The difference in the surface characteristics of the metal coping and implant could be a possible reason for its absence but since ours is a limited study with one sample, it cannot be a proven hypothesis. A study with a bigger sample size could possibly find an answer whether the surface characteristics of metal on teeth and implant influence the microflora of peri implantitis site. Thus, our study provides an overview of the peri implant microbiology and an assessment as to whether bacteria associated with periodontitis exert a possible risk for peri implant tissue breakdown. Hence microflora is the most important characteristic along with which confounding factors like occlusal trauma, parafunctional habits, implant design, the surface characteristics, the type of prosthetic appliance; all could essentially play a role in progression of peri implantitis. Thus, during treatment planning apart from the microflora even other factors should be taken into consideration for efficient management of peri implantitis. The treatment protocol for peri implantitis was suggested by Lang and Co workers which was referred to as Cumulative Interceptive Supportive Therapy (CIST)27. It includes treatment modalities that consists of Mechanical debridement, Antiseptic treatment, Antibiotic treatment (non surgical) and Regenerative or Access/ resective surgery (surgical).According to the CIST protocol, since the case dealt with in our study showed a pocket depth of 6-7mm with bone loss till the middle third of the implant body, along with Debridement and Antiseptic management, it required a Regenerative Surgical treatment with pre and post surgical antibiotic therapy.
CONCLUSION
Dental implants are increasingly common form of prosthetic device implanted into patients. The apparent high success for placement of endosseous dental implant under uncontrolled environmental conditions and through heavily colonized oral environment appears counterintuitive. Datas on failures and complication of dental implants should be collected and reported in a systematic manner. This would enable in a more detailed analysis of microbiology, treatment outcomes and assist in the formulation of clinical guidelines in implant placement and treatment of implant associated infections. There are no studies investigating the influence of a metal coping and a ball interface supported by other side with a ball abutment engaged endosseous implant which has the overdenture framework. Thus, this study might throw a light into a new beginning. Thus, it is concluded that proper periodontal infection control before the placement of dental implants in partially edentulous individuals may prevent early bacterial complications. And also continuous monitoring of partially edentulous teeth site making it infection free will help in the longevity of the implant.
ACKNOWLEGEMENT
Authors acknowledge the immense help received from the scholars whose articles are citied and included in references of this manuscript. The authors are also grateful to authors/ editors/publishers of all those articles, journals and books from where the literature for this article has been review and discussed. The authors also thank and his staff in the Department of Periodontology, SRM University for their support throughout the study.
References:
1. Albrektsson T, Zarb GA, Worthington P, Ericsson AR. The long term efficacy of currently used dental implants: a review and proposed criteria for success. Int J Oral Maxillofac Implants 1986; 1: 11–25.
2. Zarb GA, Schmitt A. The longitudinal clinical effectiveness of osseointegrated dental implants: the Toronto study. Part II: the prosthetic results. J Prosthet Dent 1990; 64: 53–61.
3. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000 1998; 17: 63–76.
4. Hultin M, Gustafsson A, Hallström H, Johansson L-Å, Ekfeldt A, Klinge B. Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implants Res 2002; 13: 349–58.
5. Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res 2002; 13: 1–19.
6. Roos-Jansåker AM, Renvert S, Egelberg J. Treatment of peri-implant infections: a literature review. J Clin Periodontol 2002; 30: 467–85.
7. Roos-Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine- to fourteen-year follow up of implant treatment: I. Implant loss and associations to various factors. J Clin Periodontol 2006; 22: 283–9.
8. Roos-Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine- to fourteen-year followup of implant treatment: II. Presence of periimplant lesions. J Clin Periodontol 2006; 22: 290–5.
9. Albrektsson T, Zarb GA, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants 1986;1:11e25.
10. Tonetti MS, Schmid J. Pathogenesis of implant failures. Periodontol 2000 1994;4:127e138.
11. Hugoson A, Norderyd O, Slotte C, et al. Distribution of periodontal disease in a Swedish adult population 1973, 1983 and 1993. J Clin Periodontol 1998;25:542–8.
12. Kees Heydenrijk, Henny J.A Meijer et al. Microbiota around Root-Form Endosseous Implants: A Review of the Literature. Int J Oral Maxillofac Implants.
13. Chen S, Darby I. Dental implants: maintenance, care and treatment of periimplant infection. Australian Dent J. 2003;48:(4):212 20.
14. Mombelli A, Lang NP. The diagnosis and treatment of periimplantitis. Periodontol 2000 1998;17:63–76.
15. Wilson TG. Not all patients are the same: Systemic risk factors for adult periodontitis. Gen Dent 1999;47:580–588.
16. Wilson GW, Nunn M. The relationship between the interleukin-1 periodontal genotype and implant loss. Initial data. J Periodontol 1999;70:724–729.
17. Kronstrom M, Svensson B, Erickson E, Houston L, Braham P, Persson GR. Humoral immunity host factors in subjects with failing or successful titanium dental implants. J Clin Periodontol 2000;27:875– 882.
18. Mombelli A, Lang NP. Microbial aspects of implant dentistry. Periodontol 2000 1994;4:74–80.
19. Armellini D, Reynolds MA, Harro JM, Molly l. Biofilm formation on natural teeth and dental implants: what is the difference? Role of Biofilms in Device-Related Infections. 2009;3:109-22.
20. Klinge B, Gustafsson A, Berglundh T. A systematic review of the effect of antiinfective therapy in the treatment of peri-implantitis. J Clin Periodontol 2002; 29(Suppl 3):213–25.PERI-IMPLANTITIS 675
21. Mombelli A, van Oosten M, Schurch E, Lang NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 1987; 2:145–151.
22. Quirynen M, Papaioannou W, van Steenberghe D. Intraoral transmission and the colonization of oral hard surfaces. J Periodontol 1996;67:986–993.
23. Rams TE, Roberts TW, Feik D, Molzan AK, Slots J. Clinical and microbiological findings on newly inserted hydroxyapatitecoated and pure titanium human dental implants. Clin Oral Implants Res 1991;2:121–127.
24. Leonhardt A, Adolfsson B, Lekholm U, et al. A longitudinal microbiological study on osseointegrated titanium implants in partially edentulous patients. Clin Oral Implants Res 1993;4:113–20.
25. Mombelli A, Marxer M, Gaberthuel T, et al. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol 1995;22:124–30.
26. Quirynen M, De Soete M, Dierickx K, van Steenberghe D. The intra-oral translocation of periodontopathogens jeopardises the outcome of periodontal therapy. A review of the literature. J Clin Periodontol 2001; 6: 499–507.
27. Lang NP, Mombelli A, Tonetti MS, Brägger U, HämmerleCH. Clinical trials on therapies for peri-implant infections. Ann Periodontol 1997;2:343–356.

|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License