IJCRR - 4(14), July, 2012
Pages: 45-49
Date of Publication: 31-Jul-2012
Print Article
Download XML Download PDF
CORRELATION OF HOMOCYSTEINE WITH MALONDIALDEHYDE AND LIPID PARAMETER IN CORONARY ARTERY DISEASE PATIENTS
Author: Sharma Anita, Sharma Ashish, Agrawal Apurva
Category: Healthcare
Abstract:Aim: To investigate the association of Homocysteine with lipid parameter and oxidative stress Methods: Population based cross sectional study included 60 coronary artery disease patients who visited Himalayan Institute of Medical Sciences during may 2008 to april 2009. Homocysteine and lipid profile was estimated by fully autoanalyzer, MDA level for oxidative stress was estimated by colorimetric technique on RA 50 Semi autoanalyzer. Statistical analysis: Pearson correlation and ?t test? from which p values were obtained. Results: Plasma level of Homocysteine and Malondialdehyde were significantly higher (p=0.000) in cases (CAD patients) than controls. Homocysteine shows significant positive correlation with Total Cholesterol, VLDA, LDL,Triglyceride and Malondialdehyde. Homocysteine shows negative but insignificant correlation with HDL. Conclusion: Levels of Homocysteine and Malondialdehyde obtained were found to be positively correlated with each other and with lipid parameters in this study. This indicates that Homocysteine enhances the oxidative stress by lipid peroxidation, which may be one of the cause for development of coronary artery disease.
Keywords: Coronary artery disease CAD), Homocysteine(Hcy), Malondialdehyde(MDA)
Full Text:
INTRODUCTION
Coronary artery disease is the commonest cause of heart disease and the most important single cause of death in the affluent countries of the world. Along with the known classical risk factors for the development of coronary artery disease (CAD) like cigarette smoking, hypertension, low HDL, diabetes mellitus, obesity and physical inactivity, few emerging risk factors have also been identified, like lipoprotein ?a‘, homocysteine, prothrombotic factor and pro- inflammatory factor [1]. Homocysteine is naturally occurring sulfur containing intermediate product in normal metabolism of methionine, an essential amino acid. Homocysteine (Hcy) is metabolized by two major pathways which require vitamin B6, B12 and folic acid [2]. Homocysteine increases the damage to the cardiovascular system in different ways, one of them is the formation of reactive oxygen species from the auto oxidation of homocysteine. The auto-oxidation of homocysteine also produces reactive oxygen species including superoxide and hydrogen peroxide and enhances oxidative stress Aldehydes such as thiobarbituric acid reacting substances (TBARS) have been widely accepted as a general marker for free radical production. The most commonly measured TBARS is malondialdehyde (MDA) [3].
The results of more than 75 clinical and epidemiological studies have indicated a positive correlation between total homocysteine levels and CAD, peripheral arterial disease, stroke, and venous thrombosis. However vary few studies have been done to established correlation between homocysteine and oxidative stress in patients of CAD. Therefore this study was undertaken with a view to establish hyperhomocysteinemia and it‘s correlation with oxidative stress as one of the risk factors for coronary artery disease.
MATERIALS AND METHOD
The present study was conducted on 60 patients of coronary artery disease over a period of 12 months (Feb 2008- Jan 2009) in the Department of Biochemistry and Cardiology of Himalayan Institute of Medical Sciences, Swami Rama Nagar, Doiwala, Dehradun. The patients were from the intensive care unit of Cardiology department at HIMS. The patients included in the study satisfied the following criteria: The study also included 30 normal age matched healthy adults (20 males and 10 females), who served as controls. Approval from ethical committee was taken prior to study. Written informed consent was taken from all the participants after explaining detailed methodology to them
Collection of samples
All samples were collected in the morning after 12 hrs of overnight fasting. 2 ml of blood was drawn by venepuncture from the antecubital vein in an EDTA vacutainer for estimation of plasma MDA and homocysteine, and 2 ml of blood was collected in a plain vacutainer for estimation of lipid profile. Estimation of plasma homocysteine, serum total cholesterol, HDL cholesterol and triglycerides were done on SYNCHRON CX5, Automated Chemistry Analyzer of Beckman Coulter Ltd. Serum LDL cholesterol and VLDL cholesterol were calculated by using Friedewald‘s formula. Estimation of plasma malondialdehyde was done by colorimetric technique on RA 50 semi automated chemistry analyzer. Cholesterol was measured by a timed end point method of Allan and Poon (1974). The concentration of triglycerides is measured by a time endpoint method of Bucco and David (1973). The homocysteine levels in the plasma was estimated using the Hcy enzymatic assay as marketed by Diazyme [4]. MDA was measured by method of ceconi,Cargoni,Pasini et al(1992).The result of all the parameters undertaken were tabulated and statistclly analysed by the Pearson correlation and ?t test? from which p value were obtained.
RESULT
Table 1 below represents the mean values obtained for lipid profile, homocysteine and MDA in the case study group as compared with control group. Levels of Triglyceride, Total Cholesterol, LDL-C, VLDL-C were higher in study group than in controls, whereas level of HDL-C was lower in the study group compared to controls. The difference is statistically significant only for TAG, VLDL-C and HDL-C. Plasma level of Hcy and MDA were significantly higher (p=0.000) in cases (CAD patients) than controls. Hcy showed significant positive correlation with Total Cholesterol, LDLC,VLDL-C and triglycerides and insignificant negative correlation with HDL-C.
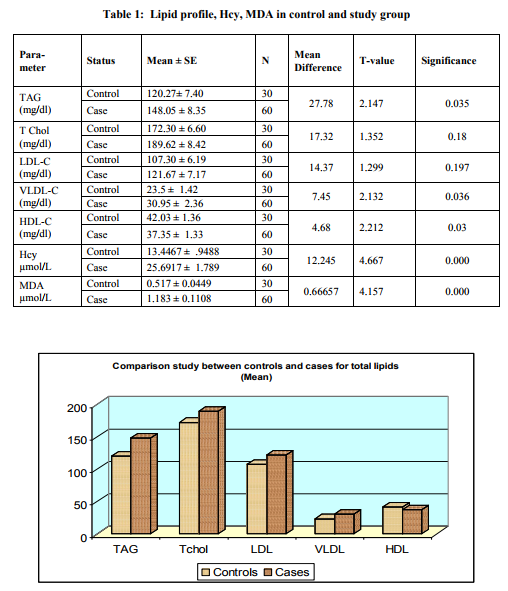
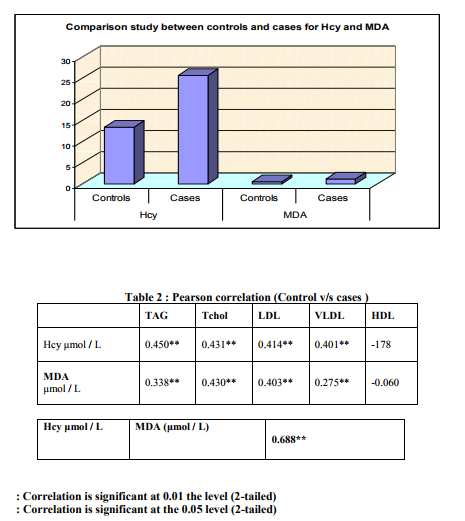
DISCUSSION
Statistically significant positive correlation between plasma Hcy and MDA obtained in our observations is supported by the study of Moselhy and Denerdash and vishnupriya and surapaneni which shows positive correlation between plasma Hcy and MDA levels with p value<0.001. Although the exact mechanism of Hcy toxicity is unknown, it is believed that tHcy or its metabolite adversely affects vascular endothelium inducing atherosclerosis by several mechanisms. e.g Auto oxidation of homocysteine generates hydrogen peroxide which has a toxic effect on endothelial cells. Hcy promotes lipids peroxidation and oxidation of low density lipoproteins. This modified oxidized LDL causes alteration of NO metabolism, alteration of functions and oxidative damages on vascular endothelial cells and platelets [4]. Auto-oxidation of homocysteine to the homocysteine radical, with the concomitant generation of superoxide anion, hydrogen peroxide, and hydroxyl radical, are the contributing factors for vascular injury that is associated with homocysteinemia . Hcy auto-oxidation and thiolactone formation promote the production of free radicals which are known to be initiators of lipid peroxidation in cells [6]. Hcy enhance lipid peroxidation. Lipid peroxidation occur more in hyperlipidemic state which can further enhance oxidative stress.
CONCLUSION
Levels of Hcy and MDA obtained were found to be positively correlated with each other and with lipid parameters in this study. This indicates that Hcy enhances the oxidative stress by lipid peroxidation, which may be one of the cause for development of coronary artery disease.
References:
1. Hackam D G, Anand S S. Emergency Risk Factors for Atherosclerosis Vascular Disease. J Am Med Assoc. 2003;290:932-40.
2. Kurban S, Mehmetoglu T, Oran B, Kiyici A. Homocysteine levels and total antioxidant capacity in children with acute rheumatic fever. Clinical biochemistry. 2007;41:26-29.
3. Tanriverdi H, Evrengul H, Enli Y, Kuru O, Seleci D, Tanriverdi S et al. Effect of Homocysteine-Induced Oxidative Stress on Endothelial Function in Coronary Slow-Flow. Cardiology 2007;107:313-320.
4. Jones B G, Rose F A, Judball N. Lipid Peroxidation and Homocysteine induced toxicity. Atherosclerosis 1994; 105: 16570.
5. Loscalzo J. The oxidant stress of hyperhomocysteinemia. J Clin. Invest. 1996; 98 : 5-7
6. Harker L A, Slichter S J, Scott C R, Ross R. Homocysteinemia. Vascular injury and arterial thrombosis. N Engl J Med 1974; 291: 537- 43.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License