IJCRR - 8(24), December, 2016
Pages: 26-32
Print Article
Download XML Download PDF
EVALUATION OF MERCURY (HG) AND ARSENIC (AS) RESIDUES IN ORGANS AND MUSCLES OF SLAUGHTERED PIGS AT NSUKKA SLAUGHTER HOUSE IN ENUGU STATE, NIGERIA
Author: Felix Chidiebere Obioha, Lynda Onyinye Obodoechi, Johnbosco Chinwuba Ukoha
Category: Healthcare
Abstract:Aim: The aim of this study was to determine the presence and concentration of mercury (Hg) and arsenic (As) in organs and tissues of slaughtered pigs in Nsukka slaughter house.
Methodology: From a total of 110 slaughtered pigs, liver, kidney and muscle samples of each pig were collected and analyzed for the detection of mercury and arsenic residue using Atomic Absorption Spectrophotometer.
Results: A prevalence rate of 66.4% and 29.1% were recorded for mercury and arsenic respectively. The level of mean concentrations of arsenic in kidney, liver and muscle were 0.0016mg/kg, 0.0025mg/kg and 0.0012mg/kg respectively. While level of mean concentrations of mercury were 0.0009mg/kg, 0.0010mg/kg and 0.0006mg/kg in kidney, liver and muscle respectively. There was a significant difference (P< 0.05) in the concentration of arsenic and mercury in liver, kidney and muscle samples of the different age groups of the slaughtered pigs.
Conclusion: The levels of mercury and arsenic in few samples that exceeded the provisional tolerable weekly intake (PTWI) may pose human health threat to pork consumers in the study area.
Keywords: Arsenic, Heavy metal, Kidney, Mercury, Residues
Full Text:
Introduction
Heavy metals are serious environmental pollutants and their uptake and accumulation in the ecosystem, beyond safe limits, would cause direct consequences to food chain and ultimately to man (Felix et al., 2016a). Heavy metals such as mercury, arsenic etc, are naturally occurring elements in the earth’s crust, and thus direct or indirect exposure to them from natural sources is inevitable especially for animals that are not intensively reared (Felix et al., 2016a). In animal tissues, metals may enter through animal feeds, green fodder, drinking water and pharmaceutical medicines etc. Other sources are accidental access to limed field, mineral supplements with high content of trace metal and licking of painted surfaced containing metallic pigments. The common source of arsenic is in fluid used for dipping and spraying of animal to control ecto-parasites. Industrial wastes and sewage water from the chloroalkali industry are a major source of mercury pollution.
Arsenic is a metal that occurs at ultra trace levels. It has been suggested that this metal could play an essential role in humans because decreases in serum arsenic concentration have been correlated with injuries of the central nervous system, vascular disease and cancer (Smith et al., 2000; Pesch et al., 2002). Chronic arsenic toxicity is mostly manifested in weight loss, capricious appetite, conjunctively and mucosal erythematic lesion including mouth ulceration and reduced milk yield. Acute toxic effects include abdominal cramping, hyperesthesia in extremities, abdominal patellar reflexes and abdominal electrocardiogram (Pesch et al., 2002).
The toxicity of mercury depends on its chemical form methyl mercury being the most hazardous metal and stable form of mercury that has been attributed to the suffering of most avian and mammalian predators at the top of contaminated tropics. Mercury has the ability to cross the blood brain barrier for example methyl-mercury causing toxicity of central nervous system in animals and as well as in humans (Mukesh et al., 2008). A well documented environmental disaster associated with mercury is the Minamata disease. Minamata disease is sometimes referred to as Chisso- Minamata disease. It is a neurological syndrome caused by severe mercury poisoning. Mercury has the ability to cross the blood brain barrier for example methyl-mercury causing toxicity of central nervous system in animals and as well as in humans. (Mukesh et al., 2008).
The polluted meats from the edible animal products exposed to heavy metals in the environment are sold in the market for human consumption (Felix et al., 2016a). Despite the high concentrations of heavy metals in Enugu State, no studies have been conducted to determine metal contamination levels in pigs which scavenge freely in the area. The animals when exposed to toxic metals accumulate them in their organs such as liver and kidneys, which are considered delicacies in Nsukka. Meat produced from these animals is a rich and convenient source of nutrients such as proteins and micronutrients.
It is therefore imperative that this study be carried out with the major aim to investigate the possible presence and prevalence of mercury and arsenic residues in organs and muscles of slaughtered pigs in the study area and also, to determine its level (concentration) in the tissues.
Materials and methods
Study Area
The study was done in Nsukka slaughter house of Enugu State, South East Nigeria. Nsukka urban has a map coordinates of 6°51′24″N and 7°23′45″E. Nsukka has a total land area of about 17.5 sq mi (45.38 km2), and has an elevation of 1,810ft (522 m) with a population of 309, 633(NPC, 2006).
Study Design
The research work was a four month cross sectional survey and laboratory analysis of samples from slaughtered pigs, to determine the presence, prevalence and concentration of Mercury and Arsenic. This experiment was conducted with the permission of the Institution’s Ethics Committee.
Sampling technique and Sample Collection
One (Nsukka) out of the three agricultural zones in Enugu State was randomly selected. Stratified random sampling was used to select pigs from the slaughter house assigning them into female and male sex strata and systematic random sampling was used to select 1 in 3 pigs slaughtered, twice a week for four months.
A total of 330 fresh samples of liver, kidney and muscle from 110 slaughtered pigs were collected between the months of June 2014 and September 2014. Age was determined using teeth eruption and wearing. About 50g each of liver and muscle samples and a whole kidney of each selected slaughter pig was packed in sterile polythene bags, labeled and sent to Veterinary Public Health and Preventive Medicine, University of Nigeria, Nsukka for freezing pending analysis. The frozen samples were transported in a cold chain to Springboard Research laboratory, Awka Anambra State, Nigeria, for chemical analysis. Information on the method of processing and the type of materials used was collected by observation and pictures were taken.
Sample processing
Digestion of Sample (Dry Digestion)
Digestion of the sample was done using the method of Felix et al. (2016a). Liver, kidney and muscle samples were dried in the oven at 45o C. After drying, individual sample was crushed into fine powder using mortar and pestle, and 1.0g of the fine powdered sample was weighed into porcelain crucible and ignited in a muffle furnace at 500o C for 6 to 8 hours. The samples were then removed from the furnace and allowed to cool in desiccators, and weighed again. 5cm cube of 1M Trioxonitrate (V) acid (HNO3) solution was added to the left-over ash and evaporated to dryness on a hot plate and returned to the furnace for re-heating at 400o C for 15-20 minutes until perfect grayish-white ash was obtained. The samples were then allowed to cool in desiccators. 15ml (cm3) hydrochloric acid (HCL) was then added to the ash to dissolve it and the solution was filtered into 100 cm3 volumetric flask. The volume was made to 100cm3 with distilled water.
Analysis
Mercury and arsenic residues were tested using the digested samples of liver, kidney and muscle under specified condition using Atomic Absorption Spectrometer (AAS). The procedure was done according to the manufacturer (AA-6800, Shimadzu Atomic Absorption Spectrophotometer) (Szkoda and Zmudzki, 2005).
Data Analysis and Presentation
The data generated from the study were statistically analyzed using SPSS version 17. Analysis of variance and post hoc test were performed to determine if there is statistical significance difference in the mean concentrations of mercury and arsenic among various age groups Descriptive statistics was also used and data generated were converted to percentages and presented in tables. P < 0.05 was considered to be significant.
RESULTS
Prevalence of mercury residue in slaughtered pigs in Nsukka slaughter house
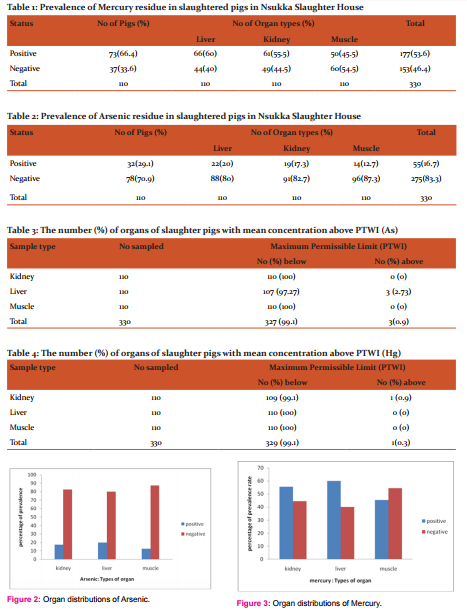
Out of a total of 110 pigs sampled, 73 (66.4%) were positive while 37 (33.6%) were negative for mercury residue and from 330 organs sampled (110 each of liver, kidney and muscle from the 110 pigs), 177(53.6%) were positive for mercury residue (Table 1).
Prevalence of Arsenic residue in slaughtered pigs in Nsukka slaughter house
Out of a total of 110 pigs sampled, 32 (29.1%) were positive while 78 (70.9%) were negative for arsenic residue and from 330 organs sampled (110 each of liver, kidney and muscle from the 110 pigs), 55 (16.7%) were positive for arsenic residue (Table 2).
Comparison of the number of positive samples and the mean Concentrations of Mercury and Arsenic in the different organs from different sources with their specific PTWI
Table 3, arsenic recorded 0 (0%) of kidney, 3(2.73%) of the liver and 0(0%) of the muscle samples respectively which were above the PTWI of 0.015 mg/kg body weight (bw). Table 4 mercury recorded 1 (0.9%) of kidney, 0(0%) of the liver and 0(0%) of the muscle samples respectively which were above the PTWI of 0.005 mg/kg bw.
Organ distributions of arsenic in slaughtered pigs
In figure (fig.) 2, the presence of arsenic was recorded 22(20%) of liver, 19(17.3%) of kidney and 14(12.7%) of muscle samples. There is no significant difference between occurrence of arsenic and the organ types.
Organ distributions of mercury in slaughtered pigs
In fig. 3, the presence of arsenic was recorded 66(60%) of liver, 61(55.5%) of kidney and 50(45.5%) of muscle samples. There is significant difference between occurrence of mercury and the organ types.
Age distribution of mercury and arsenic concentrations in Nsukka slaughter house
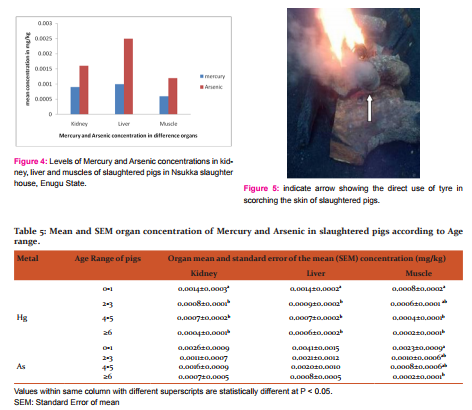
The mean mercury concentrations in age range of slaughter pigs, 0 to 1 year were 0.0014mg/kg, 0.0014mg/kg, and 0.0008mg/kg in kidney, liver and muscle samples respectively (Table 5). In age range 2 to 3 years, the mercury concentrations of 0.0008mg/kg; 0.0009mg/kg and 0.0006mg/kg were recorded in kidney, liver and muscle respectively. The mean mercury concentrations in age range 4 to 5 years were 0.0007mg/kg, 0.0007mg/kg and 0.0004mg/kg in kidney, liver and muscle respectively. The mean mercury concentrations for the age range ≥ 6 years were 0.0004mg/kg, 0.0006mg/kg and 0.0002mg/kg in kidney, liver and muscle respectively.
The mean arsenic concentrations in age range of slaughter pigs, 0 to 1 year were 0.0026mg/kg, 0.0041mg/kg, and 0.0023mg/kg in kidney, liver and muscle samples respectively (Table 5). In age range 2 to 3 years, the arsenic concentrations of 0.0011mg/kg; 0.0021mg/kg and 0.0010mg/kg were recorded in kidney, liver and muscle respectively. The mean arsenic concentrations in age range 4 to 5 years were 0.0016mg/kg, 0.0020mg/kg and 0.0008mg/kg in kidney, liver and muscle respectively. The mean arsenic concentrations for the age range ≥ 6 years were 0.0007mg/kg, 0.0008mg/kg and 0.0002mg/kg in kidney, liver and muscle respectively.
However, for arsenic and mercury residues, the European Commission has not established statutory limits for meat products. Mercury levels in our samples were very low, about 1000-fold lower than allowed in fish (European Commission, 2011). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) Provisional Tolerable Daily Intake (PTDI) for inorganic arsenic is 0.002 mg/kg bodyweight, equivalent to 0.12 mg/day for a 60kg adult. The JECFA Provisional Tolerable Daily Intake (PTDI) for inorganic arsenic is 0.002 mg/kg bodyweight, equivalent to 0.12 mg/day for a 60kg adult. PTWI for mercury is 0.005mg/kg bw and 0.0015mg/kg bw for arsenic. However, mean values for arsenic and mercury in all the organs and muscle samples were below the PTDI except in the age range 0 to 1 year (0026mg/kg, 0.0041mg/kg, and 0.0023mg/kg in kidney, liver and muscle respectively) and liver of age range 2 to 3 (0.0021mg/kg). Also, the mean values for mercury and arsenic in all the organs and muscle samples were below the PTWI except in few samples of the kidney and liver in Hg and As respectively, which was slightly higher but not statistically significant (p<0.001).
Processing of slaughter pigs in Nsukka slaughter houses
Old tyres were used to light fire in Nsukka slaughter house, for singeing slaughtered pigs (Fig 5).
Discussion
The 66.4% and 29.1% prevalence of mercury and arsenic in the study area seems significant and indicative of high exposure of pig consumers to mercury residue. The prevalence is at par with 66.7% mercury prevalence recorded by Anonymous (1989), in liver tissue of pigs at New Zealand, but slightly differs from 31.9% prevalence recorded in the kidney sample. The parity between the prevalence of mercury and arsenic residue in pigs from New Zealand and Nsukka urban abattoir in this study which recorded 66.4% prevalence could be likened to the fact that both are located in urban areas and there is regular meat inspection in the area. This may also be attributed to the higher level of industrial activities (mechanics and automobiles engines) observed in the area.
Although it has been reported by Akoto et al., 2014 that mercury and Arsenic residues accumulates more in kidney and liver as also detected in this work owing to the fact that kidney and liver are organs of biotransformation and detoxification, but no association was found between the type of tissue and the occurrence of arsenic and mercury residues respectively. The non association could be likened to the singeing practices which makes accumulation of mercury and arsenic in the muscle almost as high as in the internal organs (kidney and liver). Accumulation in internal organs (kidney and liver) occurs due to oral exposure (consumption of mercury and arsenic contaminated food and water). Animals, especially free range pigs are exposed to heavy metals in our local environment through scavenging in open waste or refuse dumps, and polluted drinking water (Okoye and Ugwu 2010; Felix et al., 2016b).
The higher levels of mercury recorded in organs in the present study is similar to what was reported in pigs slaughtered at New Zealand (Anonymous, 1989). The number of positive samples (2.73% of the liver is above the PTWI of 0.015 mg/kg body weight for Arsenic and 0.9% of kidney is above the PTWI of 0.005 mg/kg body weight for Hg) out of 110 samples of each organ, with concentrations higher than their respective PTWI as recommended. But, they values are small and the mean concentrations of the two organs in the different metals are significantly lower than their respective PTWI. The results from this study implies that Hg and As accumulates more in kidney and liver compared to muscle and is in agreement with Akoto et al. (2014). The high levels of Hg and As concentrations in liver and kidney of slaughtered pigs are likely due to their special functions; liver as storage and metabolic organ and kidney as an excretory organ (Felix et al., 2016a). The result shows that the mean concentrations of mercury and arsenic decreased as the age increased. Mean mercury concentrations in age range 0 to 1in kidney (0.0014 mg/kg), liver (0.0014 mg/kg) and muscle (0.0008 mg/kg) was significantly higher than the age range 2 years and above. Mean arsenic concentrations in age range 0 to 1in kidney (0.0026 mg/kg), liver (0.0041 mg/kg) and muscle (0.0023 mg/kg) was significantly higher than the age range 2 years and above. The reason for the variation in age is because younger pigs have immature metabolic rate and innate curiosity.
Generally, mean values for mercury and arsenic in all the organs and muscles of pigs slaughtered in the study area were below PTDI except in the arsenic age range 0 to 1 year and liver of age range 2 to 3 which was slightly higher, but the difference is not statistically significant. The fact that the mean concentration of the age range 0 to 1 is higher than its PTDI, although not significant; it may still pose serious public health threat to consumers considering the high rate of exposure recorded in this study.
These metals and metalloids tend to bioaccumulate in the environment and biomagnify in food chains where their levels might reach toxic limits even when found in low concentrations in environmental samples (Caggiano et al., 2004; Ekenma et al., 2014, Felix et al., 2016a). Serious health problems can develop as a result of excessive accumulation of these metals in the human body through dietary intakes (Oliver, 2007).
Conclusion
This study shows that heavy metals were seen in different concentrations in organs and muscles of slaughtered pigs. The level of mercury and arsenic in pigs slaughtered at Nsukka slaughter house varied with all the samples falling below the PTDI except in the arsenic age range 0 to 1 year and liver of age range 2 to 3 which was slightly higher. The results also concluded that the mercury and arsenic residues accumulates more in kidney and liver than the muscle tissue. Younger pigs are more prone to mercury and arsenic residues accumulation than the older ones.
Prolonged consumption of high concentrations of Hg and As slaughtered pigs offal and pork meat may lead to accumulation of these metals in the human body and cause metal toxicity. There is a clear need to avoid consumption of contaminated pork meat and offals. Continuous monitoring of Hg and As residues in the liver, kidney and muscle of slaughtered pigs in Nsukka slaughter house is recommended in the interest of human consumers. This study would be useful for the creation of guidelines to protect the public from the harmful effects of the toxicants present in pigs that is consumed by the public.
Acknowledgement
The authors expressed their sincere gratitude to God Almighty. Also, the authors appreciated the effort of Prof. J.A Nwanta, Mrs. Obioha Adaeze, Miss Glory Obioha and Mr. and Mrs. J.E Obioha for their encouragement, support and advice.
Finally, to all the scholars, editors and publishers whose articles were cited in our work.
Conflict of interest
There was no conflict of interest
References:
- Akoto O, Bortey-Sam N, Nakayama SMM, Ikenaka Y, Baidoo E, Yohannes YB, Mizukawa H, Ishizuka M. Distribution of Heavy Metals in Organs of Sheep and Goat Reared in Obuasi: A Gold Mining Town in Ghana. Int. J. Environ. Sci. Toxic 2014; 2(2):81-89
- Anonymous. Mercury residues in the tissues of domestic pigs and feral animals in New Zealand. Surveillance 1989; 16(1)
- Caggiano R, Macchiato MF, Ragosta M. Heavy metals in ryegrass species versus metal concentrations in atmospheric particulates in an industrial area of southern Italy. Environ Monit Assess 2004; 102(1-3): 67-84.
- European Commission (EC). Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Commission Regulation No 420/2011 of 29 April 2011 OJ L 111/3 of 30 April 2011.
- Ekenma K, Anelon NJ, Ottah AA. Determination of the presence and concentration of heavy metal in cattle hides singed in Nsukka abattoir. J. Vet. Med. Anim. Health 2014;7:9-17.
- Felix OC, Ekene E, Johnbosco UC, Anelon NJ. Assessment of cadmium (Cd) residues in organs and muscles of slaughtered pigs at Nsukka and environs in Enugu State, Nigeria. J. Vet. Med. Anim. Health 2016a; 8(11): 199-206
- Felix OC, John NA, Ekene EV. Assessment of lead (Pb) Residues in Organs and Muscles of Slaughtered Pigs at Nsukka and Environs in Enugu State, Nigeria. J Adv Vet Anim Res 2016b; Online First: 17 Nov.
- Mukesh KR, Puneet K, Manoj S, Anand S. Toxic effect of heavy metals in livestock health. Vet. World 2008;1(1):28-30
- National Population Commission (NPC). Census data. National Population Commission Nigeria.2006
- Okoye CO, Ugwu JN. Impact of environmental cadmium, lead, copper and zinc on quality of goat meat in Nigeria. Bull. Chem. Soc. Ethiop 2010; 24(1):134.
- Oliver MA. Soil and human health: a review. Eur J Soil Sci 2007; 48: 573-92
- Pesch B, Ranft U, Jakubis P, Nieuwenhuijsen MJ, Hergemoller A, Unfried K, Jakubis M, Miskovic P and Keegant T. Environmental arsenic exposure from a coal-burning power plant as a potential risk factor for nonmelanoma skin carcinoma: results from a case-control study in the district of Prievidza, Slovakia. Am. J. Epidemiol 2002;155: 798–809.
- Smith AH, Arroyo AP, Mazumder DN, Kosnett MJ, Hernandez AL, Beeris M, Smith M M and Moore LE. Arsenic induced skin lesions among Atacamano people in Northern Chile despite good nutrition and centuries of exposure. Environ. Hlth. Perspectives 2000;108: 617–620.
- Szkoda J O, Zmudzki J. Determination of lead and cadmium in biological material by graphite furnace atomic absorption spectrometry method. Bull. Vet. Inst. Pulawy 2005;49:89-9
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License