IJCRR - 5(11), June, 2013
Pages: 80-86
Date of Publication: 18-Jun-2013
Print Article
Download XML Download PDF
A COMPARATIVE EVALUATION OF HBA1C MEASUREMENT IN DIFFERENT ANTICOAGULANT VIALS AND ITS STABILITY ON STORAGE
Author: Devajit Sarmah, Booloo Sharma
Category: Healthcare
Abstract:Introduction: India has an overwhelming diabetic population and the importance of HbA1c testing is felt. However the need of an additional EDTA sample and due to lack of standardised laboratories in most part of India, samples are stored and transported for testing. The study aims to check the variation in HbA1c measurement in K3EDTA, Na-citrate, lithium-heparin and Na-fluoride/Na2 EDTA anticoagulant vials and check for its stability when sample are stored at 2 \? 8\?C for 7 days. Methods and materials: HbA1c was measured by cation exchange HPLC based BioRad D - 10 analyser after collection of sample in K3EDTA, Na-citrate, lithium-heparin and Na-fluoride/Na2 EDTA from 25 diabetic and 25 non - diabetic subjects on day 1 and on day 3, day 5 and day 7 of sample collection, after storage at 2 \? 8\?C. Result: There were no variation in the measured value of HbA1c in these anticoagulant vials (CV = 0.0 \? 0.8%). There were no significant differences in the mean values of the HbA1c measured on day 1 and day 7 (8.2 \? 1.16 vs. 8.2 \? 1.18, P = 0.962 in diabetic and 5.3 \? 0.25 vs. 5.3 \? 0.24, P = 0.955 in non - diabetic). There were significant correlations between the HbA1c values measured on day 1 and after 7 days storage (r = 0.991 in diabetic and r = 0.957 in non \?diabetic). Conclusion: HbA1c values in fresh and stored whole blood sample does not change when analysed in K3EDTA, Na-citrate, lithium-heparin and Na-fluoride/Na2 EDTA anticoagulant vials.
Keywords: HbA1c, K3EDTA, Na-citrate, lithium-heparin and Na-fluoride/Na2 EDTA, prolonged storage.
Full Text:
INTRODUCTION
Worldwide the prevalence of type-2 diabetes mellitus (T2DM) has been rising, and about 90 % of the diabetic populations are of T2DM. Of 371 million diabetic people worldwide, 63 millions are Indian, i.e. every sixth diabetic is an Indian, as reported by the International Diabetic Federation (IDF) 2012 report (1). The dramatic economic changes have had a great impact on urbanization and lifestyle of the Indians, which together with genetic predisposition contributed to the rise in diabetes in India (2). Also the presentation of T2DM occurs a decade earlier in Indians when compared to European population (3). Traditionally, for diagnosis of diabetes physicians rely on fasting plasma glucose (FPG) and oral glucose tolerance test (OGTT). Glycated haemoglobin (HbA1c) was done only in a few numbers of cases and it was used only for monitoring the effect of treatment. HbA1c is always considered as a stable indicator of glycaemia for the preceding three months (4). Its potential utility in diabetic care was first reported in 1985 World Health Organisation report (5), and by 2010 all the major expert committee and association across the globe including the American Diabetes Association (ADA) has recommended HbA1c for the diagnosis of Type 2 DM, besides its role in prognosis (6). So, the importance of HbA1c estimation in diabetes has increased manifold in recent years. Most of the commercial kits for HbA1c estimation requires sample to be collected in EDTA anticoagulant. This requires additional sample collection for the patient. So, we estimated HbA1c in blood sample collected in K3EDTA, Na-citrate, lithium-heparin and Nafluoride/Na2 EDTA vials separately and then we checked for any variation in HbA1c values. Also most laboratory uses fresh sample for estimation of HbA1c. As standardized methods for HbA1c estimation like high performance/pressure liquid chromatography (HPLC) etc. are not available in all the laboratories in India, so samples are to be stored and transported to a standardised laboratory for estimation. Thus, the sample requires storage until it is being analysed. It is said that HbA1c has high pre-analytical stability and is stable for 1 week when stored at 4°C and for 1 year when stored at -70°C (7, 8). So, to verify the stability of HbA1c in the above vials when stored at 2 - 4°C, HbA1c was estimated for 7 days in all the different anticoagulant vials, in context to Indian settings.
MATERIAL AND METHODS
The study was conducted from June 2012 to October 2012 in the Central Clinical Laboratory of the R D Gardi Medical College in Ujjain, India. A total of 25 adult Type 2 diabetic cases (diagnosed by ADA criteria or known cases) of either sex in the age group of 25 to 65 years, and 25 adult healthy non - diabetic patients in the same age group of either sex were considered for the present study. Only those subject who were not having any disease other than diabetes that may affect the HbA1c values were selected for the study. They were excluded based on necessary patient history and investigations. Clearance from the ethical committee and consent from the study subjects were obtained. K3EDTA, Na-citrate, lithium-heparin and Nafluoride/Na2 EDTA vacutainer vials manufactured by Becton, Dickinson (BD) India, were used. The creation of the two groups is simply to ascertain the purpose of the study in samples with a normal and a higher HbA1c values separately. 2ml of venous sample were drawn in all the four different vacutainers from each of the diabetic patients and healthy individuals, using standard venipuncture techniques. HbA1c was estimated after 2 hours of blood collection by cation exchange HPLC based BioRad D - 10 dual programme analyzer, manufactured by BioRad, USA. Just after estimation, vials were stored at 2 - 8 oC and then HbA1c measured every alternate day till 7th day in all the vials i.e. on 1st(day of sample collection), 3rd, 5th and 7th day. The results were expressed as mean ± SD. The difference between the initial HbA1c values and the values after storage were determined using paired student’s t-tests while the correlation between the initial HbA1c values in the different anticoagulant vial and the values after storage were determined by Pearson correlation technique. A P-value < 0.05 was considered statistically significant on two-tailed testing for all analysis.
RESULTS
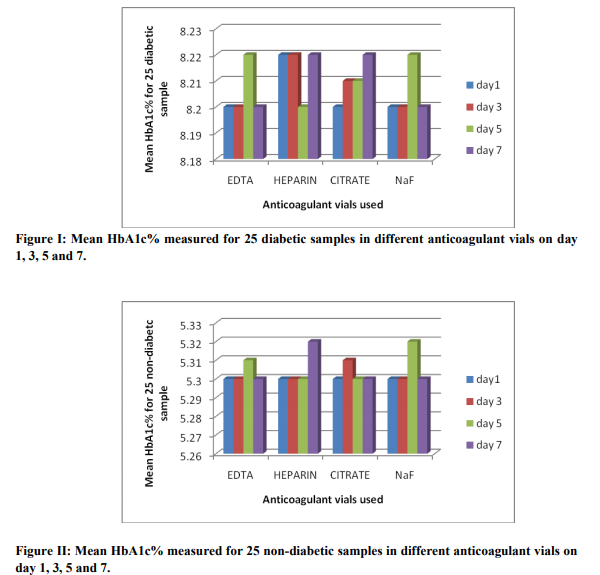
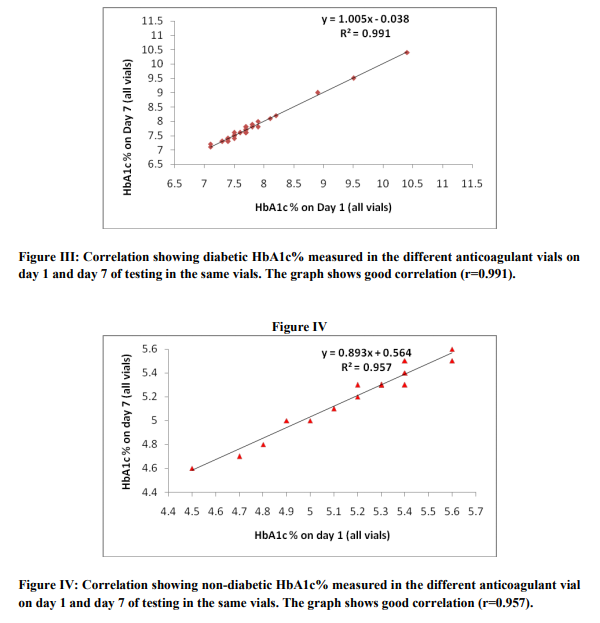
The present study shows no significant change in the measured value of HbA1c when it was measured using vacutainers containing different anticoagulant mentioned above in both the groups, i.e., the group with a normal HbA1c (non-diabetic) and the group with a higher HbA1c values(diabetic). The measured range of HbA1c value in diabetic group was from 7.1% to 11.3%. The mean HbA1c being 8.2% in this group. The range of HbA1c value in non-diabetic group was from 4.5% to 5.6%. The mean HbA1c being 5.3% in this group. The first day (day 1) estimated HbA1c value in the four different anticoagulant vials for the 25 diabetic samples were expressed as mean ± SD. The mean ranges from 7.1 – 11.3% and the SD ranges from ± (0.00 – 0.06), hence the variation was insignificant. The coefficient of variation for the 25 diabetic samples measured on day 1 among four different anticoagulant vials ranges from 0.0 to 0.8%. In the non-diabetic group the measured HbA1c value for day 1 when expressed as mean ± SD, the mean ranges from 4.5 – 5.6% and SD ranges from ± (0.00 – 0.06). Also the coefficient of variation for the 25 non - diabetic samples measured on day 1 among four different anticoagulant vials ranges from 0.0 to 0.8%. Similar results were found for the HbA1c values measured in the four different anticoagulant vials among both the diabetic and non - diabetic group on day 3, day 5 and day 7. The study therefore proves that there was no variation in measurement of HbA1c when it was measured in K3EDTA, Na-citrate, lithium-heparin and Nafluoride/Na2 EDTA vacutainer vials. Figure I and II shows the mean HbA1c measured in different anticoagulant vials among the 25 diabetic samples and non-diabetic samples respectively on day 1, day 3, day 5 and day 7 of testing. The figures depicts that the mean HbA1c varies from 8.2 – 8.22% and 5.3 – 5.32% for diabetic and non-diabetic samples respectively. When the HbA1c was measured in all the four anticoagulant vials on day 3, day 5 and day 7, the mean HbA1c value did not vary in both the diabetic and non - diabetic group. There was no significant differences in the mean values of the HbA1c measurement on day 1 and the values obtained after storage for 7 days (8.2 ± 1.16 vs. 8.2 ± 1.18 in diabetic and 5.3 ± 0.25 vs. 5.3 ± 0.24 in non - diabetic), and the P value being 0.962 and 0.955 for the diabetic group and non - diabetic group, respectively. Figure III and IV shows the correlation between the HbA1c measurement on day 1 and day 7 in the diabetic and non - diabetic group, respectively. There were significant correlations between the HbA1c values measured on day 1 and day 7 after storage in both the diabetic group (r=0.991) and the non - diabetic group (r=0.957). Also the inter assay coefficient of variation for the day 1 and day 7 sample in the diabetic and the non - diabetic group was 14.0 % and 4.6 %, respectively. The study therefore proves that there was no variation in measured values of HbA1c after storage at 2 – 8 oC for 7 days irrespective of the collection of sample in K3EDTA, Na-citrate, lithium-heparin or Na-fluoride/Na2 EDTA vacutainer vials.
DISCUSSION
The study was conducted to determine the variation of HbA1c value when measured in different anticoagulant vials as against only EDTA advocated by most manufacturers. The study also determines the stability of HbA1c in the different vials when sample were stored at 2 - 8 oC for 7 days. This study infers that there was no significant variation in the HbA1c values when it was measured in K3EDTA, Na-citrate, lithiumheparin and Na-fluoride/Na2 EDTA vacutainer vials. The study also confirms the stability of HbA1c measurement in the above vials when the samples were stored at 2 - 8 oC for 7 days. According to 2012 IDF report there are about 54% undiagnosed diabetic in India (1). When it comes to awareness in India only 1/3rd of diabetics under ImproveTM study were aware of HbA1c (9). Even today HbA1c is a very rare test in India, which is because of the cost and lack of standardised laboratory in most part of the country (10). Very recently there is an increase in the numbers of HbA1c test request by the treating physicians. India is a developing economy where almost 70% of the population resides in the rural India. There are seldom any standardised and well-equipped laboratories in rural areas, and sample for testing are often transferred to a nearby town or city for testing after being collected in the rural areas. Sometime even a well-equipped laboratory in remote town has to wait for reagent supply. In these cases the sample for testing has to be stored until it could be analysed. It is realised that HbA1c testing is costly and the use of a separate EDTA vacutainer for testing increases the cost further. Often fasting sugar and HbA1c testing are done on the same day, but sample are collected in a Na-fluoride/Na2 EDTA vial for fasting sugar and an EDTA vial for HbA1c resulting in requirement of more sample volume and extra cost. So, considering these facts our present study seems to very promising when it comes to HbA1c testing. Our study clearly discourages the need for an extra EDTA vial for HbA1c testing, which can be tested in the same Na-fluoride/Na2 EDTA vial used for fasting / post prandial sugar, or in the same K2 EDTA vial used for complete blood counts (CBC), or in the same heparin vial in ICU patient used for blood gas analysis. The cost incurred in HbA1c testing will be reduced, the compliance will increase and this in turn will contribute to a better management of diabetes. Our study also reduces the apprehension of the treating physicians regarding the stability of HbA1c test, which are transported to standardised laboratory quite far from the place of sample collection. Physicians can now rely on the reports of HbA1c received and this in turn will generate faith on HbA1c testing in India, resulting in increase request for HbA1c testing. This is very important for a country like India where more people suffer from diabetic complications due to lack of proper monitoring, especially when diabetic complications are found to occur early in India (10, 11, 12). India is a country where proper diabetic screening and adequate manpower like dieticians and field workers for diabetic awareness is lacking, and so the importance of HbA1c testing is even more in India. We also think that HbA1c should also be used for diagnosis of diabetes in India because this could diagnose many cases which go undetected by routine fasting sugar analysis. But before this a pan India HbA1c study should be conducted to establish a base line HbA1c level for Indians, based on which diagnosis and screening could be practiced (13). Until then HbA1c should be done in almost all cases for monitoring of diabetes and in selected cases for diagnosing diabetes in India. Lack of standardised laboratories is a matter of concern and transportation of sample is widely practiced and we see that our study will help gain confidence of patient and treating physician in HbA1c testing in India. HbA1c is very important in diagnosing and monitoring diabetic in a country like India which is considered the diabetic capital of the world. CONCLUSION Our study shows that HbA1c can be measured any of the K3EDTA, Na-citrate, lithium-heparin and Na-fluoride/Na2 EDTA vials without any change in the measured value. So no extra vial is required and which saves lot of money. It also shows that when appropriate cold storage is maintained the sample remains stable for seven days, which is especially important when samples are to be transported for estimation in a place far from the place of collections.
ACKNOWLEDGEMENT
We are thankful to Dr. V. K. Mahadik, Medical Director of Ujjain Charitable Trust for providing a BioRad D-10 analyser and encouraging us for research in the field of diabetes diagnosis and screening. Authors acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors are grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
References:
1. International diabetic federation, IDF Diabetic atlas, 2012, 5th edition. Available at: www.idf.org . Accessed December 20th 2012.
2. Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian Scenario. Indian Journal of Medical Research 2007; 125: 217-30.
3. Mohan V, Venkatraman JV, Pradeepa R. Epidemiology of cardiovascular disease in type 2 diabetes: The Indian scenario. J Diabetes Sci Technol 2010; 4:58–70.
4. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Eng J Med 1993; 329(14):977- 86.
5. Diabetes Mellitus : Report of a WHO study Group, Technical Report Series 727, Geneva, World Health Organisation, 1985.
6. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012; 35 (Suppl 1):S64–S71.
7. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M.Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002; 48:436–472.
8. Little RR, Rohlfing CL, Tennill AL, Connolly S, Hanson S. Effects of sample storage conditions on glycated hemoglobin measurement: evaluation of five different high performance liquid chromatography methods. Diabetes Technol Ther 2007; 9:36– 42.
9. Shashank R Joshi, AK Das, VJ Vijay, V Mohan Challenges in Diabetes Care in India: Sheer Numbers, Lack of Awareness and Inadequate Control. JAPI, 2008; 56:443-450.
10. Joshi SR, Das AK, Vijay VJ, Mohan V. Challenges in diabetes care in India: sheer numbers, lack of awareness and inadequate control. J Assoc Physicians India 2008; 56: 443-50.
11. Mohan V, Venkatraman JV, Pradeepa R. Epidemiology of cardiovascular disease in type 2 diabetes: The Indian scenario. J Diabetes Sci Technol 2010; 4:58–70.
12. Raheja BS, Kapur A, Bhoraskar A, Sathe SR, Jorgensen LN, Moorthi SR, et al. Diab Care Asia-India Study: diabetes care in India - current status. J Assoc Physicians India 2001; 49: 717-22.
13. Sarmah D, Sharma B. Importance and Status of HBA1C in T2DM and its Indian Perspective. Asian Journal of Biomedical and Pharmaceutical Sciences 2012; 2 (12), 1-10.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License