IJCRR - 5(23), December, 2013
Pages: 63-69
Date of Publication: 16-Dec-2013
Print Article
Download XML Download PDF
BIOCHEMICAL ASSESSMENT OF LIVER DAMAGE IN SMOKELESS TOBACCO USERS
Author: Velayutharaj Alwar, Ramesh R., Niranjan G., Chandrahas Kala
Category: Healthcare
Abstract:Smokeless tobacco is as dangerous as cigarette smoking. In fact, smokeless tobacco is equally addictive and carcinogenic. Damage to the antioxidant defence mechanism is noted in smokeless tobacco (ST) users. Even drug doses have to adjust to smokeless tobacco users as it affects the detoxifying capacity of the liver. We need more studies in south Indian population involving liver enzymes and oxidative stress parameters in ST users. Hence, we planned our study to find out whether ST induced liver damage can be detected in the initial stages by estimating the liver enzyme and MDA levels. In this study we included 30 individuals who were smokeless tobacco chewers and healthy controls. It was a prospective case control study performed in adult males between the ages 30 - 50 years. Subjects: Smokers and alcoholics were excluded from this study. Serum ALT, AST, ALP, GGT and MDA levels were estimated and compared using un-paired students t test between the two groups. The liver enzymes namely AST, ALT and ALP and GGT were significantly higher than healthy controls with a `p' value < 0.05. MDA level was significantly higher in ST chewers than healthy controls with a `p' value < 0.05. It is imperative to monitor liver enzymes in order to create an awareness on the hazards of using smokeless tobacco products among the Indian population. Smokeless tobacco is used has an alternative to cigarettes by poor people as it is less costly. Avoidance of tobacco chewing could avert many cancer deaths in India.
Keywords: smokeless tobacco (ST) users, MDA, ALT, AST, ALP, GGT levels, Puducherry.
Full Text:
INTRODUCTION
Smokeless tobacco is being made available in many forms and cheaper. It is easily available and used by literates and illiterates alike in India, as an alternative to smoker's tobacco (1). Smokeless tobacco consumption is a significant source of morbidity and mortality in India (2). As per survey (Global Adult Tobacco Survey), across 16 countries, 3 billion peoples are using tobacco products in different forms (3). According to the survey, in India smokeless tobacco use is more compared to other countries in the world. In India, Smokeless tobacco products have been in existence for thousands of years among different populations. Over time, these products have gained popularity throughout the world. The term chewing tobacco is often associated with dipping tobacco (split tobacco, moist snuff) where users place a dip of tobacco between the lower or upper lip and gum by resting the dip on the inside lining of the mouth.
Once considered a harmless pleasure of gaining euphoria smokeless tobacco is now considered as dangerous as cigarettes and even more addictive (4) .Damage to the antioxidant defence mechanism is noted in smokeless tobacco users (5) . We need
more studies in south Indian population involving liver enzymes and oxidative stress parameters in ST users and its effect on liver functions have to explore.
Hence, we planned our study to find out whether ST induced liver damage can be detected in the initial stages by estimating the liver enzymes and MDA levels
Review of literature
Every year, the use of tobacco products causes a heavy toll of deaths and severe human disease worldwide. Smokeless tobacco products (STP) are used without combustion and this eliminates the danger of direct exposure of toxic combustion compounds to the lung and other tissues of the user and of the people around. But the use of STP may result in other health hazards, local or systemic according to the manner of administration and to the content of various toxic products, including nicotine and tobacco-specific nitrosamines (7). Smokeless tobacco comes in two main forms: snuff and chewing tobacco. In India, the use of domestic types of chewing tobacco is a major cause of oral cancer and is also harmful during pregnancy. Different ways in which ST is used are Gutkha, Pan Masala, Zarda, etc. and other forms. The majority of commercial tobacco products use N. Tabacum species
Nicotine is psychoactive ingredient, is metabolically inactivated by CYP2A6 to cotinine (8). Nicotine is metabolized by the liver and detoxified (9). Tobacco contains more than 2,500 documented chemical constituents, including chemicals applied to tobacco during cultivation, harvesting, processing (10). Chewing tobacco contains more than two dozen cancer causing ingredients; chewing tobacco contains three to four times more nicotine than that delivered by a cigarette and it stays for a longer time in the bloodstream.
The major tobacco alkaloid nicotine and its principal metabolite cotinine are metabolized to pyridine-N-glucuronides, Nicotine-N-Gluc and cotinine-N Gluc in the liver(11). Nicotine also inhibits antigen mediated signalling in T-cells and this block the proliferation and differentiation of lymphocyte and suppression of antibody forming cells. There is an increased production of pro-inflammatory cytokines (IL-1,IL-6 and TNF-à)which are involved in liver cell injury (12). Studies have shown that there is a decrease in antioxidant enzymes [hepatic glutathione (GSH), glutathione peroxidase (GPx), Super oxide dismutase (SOD) and catalase (CAT)] and increased lipid peroxidation (Lpx)(13). These factors lead inflammation of liver (14). This depends on the concentration and duration of STs use. There are reports revealing hepatic lipid peroxidation induced damage to the DNA causing mutation which later may lead to hepatocellular carcinoma in patients using STs.
Hence, it is imperative that liver function test and oxidative stress level is monitored in these smokeless tobacco users and to advise regarding hazards of smokeless tobacco.
Aims
To assess the degree of liver damage and antioxidant status in smokeless tobacco users
Objectives
To estimate liver enzymes such as AST (Aspartate transaminase), ALT (Alanine transaminase), ALP (Alkaline phosphatase) and Gamma Glutamyl Transferase GGT) in smokeless tobacco users and to compare with normal healthy controls.
To estimate the levels of MDA (Malondialdehyde) in smokeless tobacco users and compared with normal healthy subjects.
MATERIALS AND METHODS
We collected a total of 60 samples which were divided into two (1) Smokeless tobacco chewers and (2) Controls. Cases [n=30] :This was a Prospective case control study performed in adult males between the ages 30 - 50 years who had visited a tertiary care hospital in Puducherry, during the months of May and June 2012. All these cases
were collected from smokeless tobacco users. Controls [n=30] Age and gender matched healthy controls were included in our study. Healthy controls having the same dietary habits were selected from those who had no previous history of using smokeless tobacco in any form.
Exclusion criteria: [For both control and cases groups]
Criteria Reasons : Males <30 years and >50 years of age The age where addiction is less Female subjects Hormonal disturbance due to the menstrual cycle that may alter the values. Patients with liver pathology (e.g.: Viral Hepatitis, post hepatic jaundice) , chronic clinical conditions, metabolic and kidney disorder , and smokers as they cause liver damage and the values may be altered . Males who consume alcohol >2 drinks per day* Alcohol consumption causes liver damage and alters values especially GGT.
(* One drink - one 12-ounce bottle of beer or wine cooler, 1.5 ounces).
Estimation of biochemical parameters
Five ml of venous blood was collected from the subjects after obtaining proper written informed consent. The following biochemical parameters were estimated based on established spectrophotometric and automated procedures, approved by the International Federation of Clinical Chemistry and Laboratory medicine (IFCC)
ALT and AST were estimated using kinetic methods based on liquid stable reagents
GGT was estimated by carboxysubstrate method using a liquid stable GGT kit
Malondialdehyde (MDA) was estimated spectrophotometrically using the TBARS assay kit.
The Ethical Clearance - Protocol was duly submitted to the Institutional Human ethics committee and approval was taken before starting the study. All the procedure was informed to the patient in his native language and informed written consent was taken from them.
Statistical analysis: All the data were expressed as mean+/- SD; unpaired Students t- test was used to compare the data between the two groups of smokeless tobacco chewers and healthy control. A p value <0.05 was statistically considered significant for all statistical tests. SPSS package was used for all statistical analysis.
RESULTS
In our study, we collected a total of 60 samples which were divided into two groups-Smokeless tobacco chewers and healthy controls
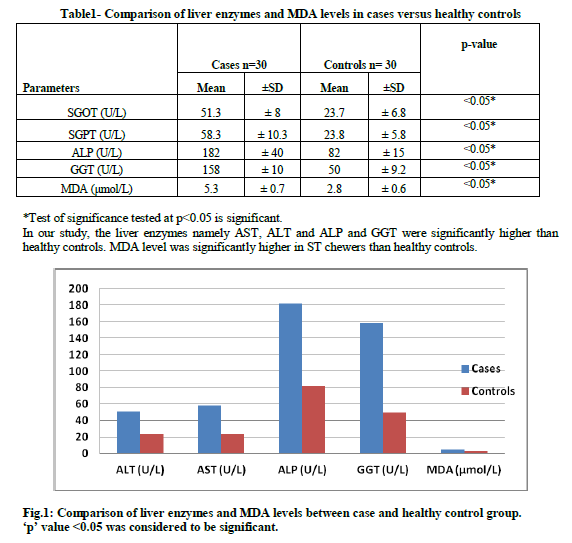
Table1- Comparison of liver enzymes and MDA levels in cases versus healthy controls. (Cases n=30 Controls n=30). In our study, the liver enzymes namely AST, ALT and ALP and GGT were significantly higher than healthy controls. MDA level was significantly higher in ST chewers than healthy controls.
Fig.1: Comparison of liver enzymes and MDA levels between case and healthy control group p' value <0.05 was considered to be significant.
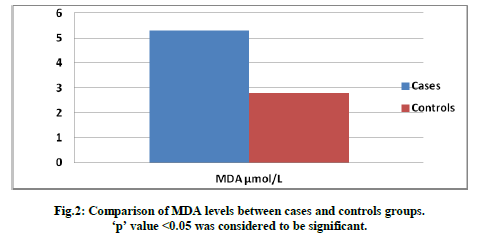
Fig.2: Comparison of MDA levels between cases and controls groups p' value <0.05 was considered to be significant.
DISCUSSION
We noticed a significant increase in MDA levels in ST users as compared to the healthy controls
A study performed earlier on the effect of nicotine, envisaged increased levels of MDA , ALT and AST level. They also observed a decline in antioxidant enzymes, Superoxide dismutase (SOD) and catalase activity (16). This study strongly depicts the fact that nicotine exposure induces oxidative stress on liver.
One more study from Kochi has shown that erythrocyte MDA levels were at significantly increased and with a significant decrease in SOD and glutathione reductase (GR) activity (17). Hence, this study has shown that, smokeless tobacco causes a duration dependent increase in oxidative stress. Similarly, we found that there
was a significant increase in ALT and AST levels pertaining to ST users, as compared to healthy controls. One previous histo-pathological study done in rat, clearly indicated that the administration of AEST (aqueous extract of smokeless tobacco) at high doses for 2 weeks in the rat could cause inflammation of the liver parenchyma and decrease in liver antioxidant enzyme status such as glutathione (GSH), glutathione peroxidase (GPx), SOD and CAT(18) .
There is an increased production of pro-inflammatory cytokines (IL-1,IL-6 and TNF-à) which are involved in liver cell injury (19).In our study, we observed a significantly raised GGT level (gamma glutamyl transpeptidase) A previous study demonstrates the effect of smoking on GGT levels and pointed to significant interaction concerning ALT and AST levels and this interaction was the same in direction with GGT levels. GGT level is basically increased and may be due to hepatocellular vulnerability and injury (20).
In our study, we observed a significantly raised ALP levels in smokeless tobacco users as compared to healthy controls. No previous study available showing comparisons of ALP levels in smokeless tobacco chewers versus healthy controls. So, in our study, we found that there is a significant alteration in liver enzymes and oxidative stress parameter MDA in smokeless tobacco chewers as compared to healthy controls.
CONCLUSIONS
In conclusion, our study might be helpful in creating awareness on the hazards of using smokeless tobacco products, among the Indian population who are using smokeless tobacco products as a cheap alternative to tobacco smoke. Avoidance of tobacco chewing could avert many cancer deaths in south India and more so, specifically in Puducherry region.
Limitations
In our study, due to time constraints, it was not possible to study the dose and duration dependent increase in oxidative stress and liver enzyme status in smokeless tobacco users. Hence, further study is required to establish dose and duration dependent effects of nicotine in smokeless tobacco users.
Implications
It should be mandatory to perform a liver enzyme parameter study in smokeless tobacco users before starting any new drug which is detoxified by the liver .Smokeless tobacco diminishes the liver's ability to detoxify dangerous substances and affects the dose of medication required.
We suggest that the use of antioxidants in subjects who are willing to quit ST will help them in preventing the imminent damage to liver and possibly other organs in the body. But for this we require further study of antioxidant use and their effects on liver enzymes and oxidative stress parameters after ST quit.
ACKNOWLEDGEMENTS
The Authors are thankful to the Institution, Head of the Departments, Technical staff and other Scholars who helped to carry out the present work. The authors acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and where the literature for this article has been reviewed and discussed. The authors are grateful to the IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
References:
- R, Suresh K, Sasikala K, Kumar BL, Venkatesan R, Ganesh GK, et al. Genotoxicity assessment in smokeless tobacco users: a case-control study. Toxicol IND Health. 2013 Mar;29(2):216-23.
- Zhou J, Michaud DS, Langevin SM, McClean MD, Eliot M, Kelsey KT. Smokeless tobacco and risk of head and neck cancer: Evidence from a case-control study in New England. Int J Cancer. 2013 Apr 15;132(8):1911-7.
- Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: a nationally representative cross-sectional household surveys. The Lancet, Volume 380, Issue 9842, Pages 668 - 679, 18 August 2012
- Phillips CV, Guenzel B, Bergen P. Deconstructing anti-harm-reduction metaphors; mortality risk from falls and other traumatic injuries compared to smokeless tobacco use. Harm Reduction Journal 2006, 3:15
- Philips CV, Wang C, Guenzel B. You might as well smoke; the misleading and harmful public message about smokeless tobacco. BMC Public Health.2005; 5:31.
- Patel BP, Rawal UM, Dave TK, Rawal RM, Shukla SN, Shah PM, et al. Lipid peroxidation, total antioxidant status, and total thiol levels predict overall survival in patients with oral squamous cell carcinoma. Integr Cancer Ther. 2007 Dec;6(4):365-72.
- Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB J. 2012 Dec;26(12):4778-87
- Zhu AZX, Bennington MJ, Renner CC, Lanier AP, Hatsukami DK, Stepanov I, et al.Alaska Native smokers and smokeless tobacco users with slower CYP2A activity have lower tobacco specific nitrosamine bioactivation .Carcinogenesis.2013Jan;34(1):93-101.
- Raunio H, Rahnasto-Rilla M. CYP2A6: genetics, structure, regulation, and function. Drug Metabol Drug Interact. 2012 May 5;27(2):73-88.
- Borgerding MF, Bodnar JA, Curtin GM, Swauger JE. The chemical composition of smokeless tobacco: A survey of products sold in the United States in 2006 and 2007. Regul Toxicol Pharmacol. 2012 Dec;64(3):367-87
- Marclay F, Saugy M. Determination of nicotine and nicotine metabolites in urine by hydrophilic interaction chromatography-tandem mass spectrometry potential use of smokeless tobacco products by ice hockey players.J Chromatogr A.2010 Nov.26;1217(48):7528-38.
- Yanagita M, Kobayashi R, Kojima Y, Mori K, Murakami S. Nicotine modulates the immunological function of dendritic cells through peroxisome proliferator-activated receptor-? upregulation. Cell Immunol. 2012;274(1-2):26-33
- Bagchi M, bagchi D, Hassoun EA, Stohs SJ. Smokeless tobacco induced increases in hepatic lipid peroxidation, DNA damage and excretion of urinary lipid metabolites. Int J Exp Pathol.1994 June; 75(3):197-202.
- Mitchell C, Joyce AR, Piper JT, McKallip RJ, Fariss MW. The role of oxidative stress and MAPK signaling in reference moist smokeless tobacco-induced HOK B cell death. Toxicol Lett. 2010 May 19;195(1):23-30.
- Restivo FM, Laccone MC, Buschini A, Rossi C, Poli P. Indoor and outdoor genotoxic load detected by the Comet assay in leaves of Nicotiana tabacum cultivars Bel B and Bel W3. Mutagenesis. 2002 Mar;17(2):127-34.
- Halima BA, Sarra K, Kais R, Salwa E, Najoua G. Indicators of oxidative stress in weanling and pubertal rats following exposure to nicotine via milk. Hum Exp Toxicol. 2010 Jun;29(6):489-96.
- Samal IR, Maneesh M, Chakrabarti A. Evidence for systemic oxidative stress in tobacco chewers. Scand J Clin Lab Invest. 2006;66(6):517-22.
- Avti PK, Kumar S, Pathak CM, Vaiphei K, Khanduja KL. Smokeless tobacco impairs the antioxidant defense in liver, lung, and kidney of rats. Toxicol Sci. 2006 Feb;89(2):547-53.
- Petro TM, Schwartzbach SD, Zhang S. Smokeless tobacco and nicotine bring about excessive cytokine responses of murine memory T-cells. Int. J Immunopharmacol. 1999.Feb;21(2):103-14.
- Breitling LP, Arndt V, Drath C, Brenner H. Liver enzymes: interaction analysis of smoking with alcohol consumption or BMI, comparing AST and ALT to GT. PLoS ONE. 2011;6(11):e
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License