IJCRR - 7(16), August, 2015
Pages: 78-85
Date of Publication: 21-Aug-2015
Print Article
Download XML Download PDF
CLINICO HISTOPATHOLOGICAL STUDY OF UPPER GASTROINTESTINAL TRACT ENDOSCOPIC BIOPSIES
Author: Syed Imtiyaz Hussain, Ruby Reshi, Gulshan Akhter, Ambreen Beigh
Category: Healthcare
Abstract:Background: Upper gastrointestinal tract is an important site for wide variety of lesions especially malignant tumors. Endoscopy in combination with endoscopic biopsy plays an important role in the diagnosis of upper gastrointestinal tract neoplasms and therefore aids in their early management. Aim: To study the histopathology of upper Gastrointestinal endoscopic biopsies and correlate them with clinical presentation, age, sex and to find the density of Helicobacter pylori in gastritis, gastric ulcers and duodenal ulcers. Methodology: A two and a half year observational study was carried out on 132 upper Gastrointestinal endoscopic biopsies. Endoscopic upper Gastrointestinal biopsies were fixed in 10% formalin overnight before processing. Routine Hematoxylin and Eosin stain and a special stain Giemsa was done to detect H. pylori. Results: Out of total 132 upper Gastrointestinal endoscopic biopsies, 37 were from esophagus, 13 from Gastrointestinal junction, 75 from stomach and 7 from Duodenum. Squamous cell carcinoma was more common in esophagus (89%), adenocarcinoma in Gastrointestinal junction (61.5%) and Gastritis in 56% of patients. Dysphagia was common symptom in patients of Squamous cell carcinoma (100%), epigastric pain in patients of adenocarcinoma (42.9%), dyspepsia in gastritis patients (71.4%). Squamous cell carcinoma, adenocarcinoma and gastritis was more common in the age group of 41-60 years with male predominance (66.7%, 84.6% and 69% respectively). Helicobacter pylori gastritis was present in 32 cases (76.1%) while Helicibacter pylori negative gastritis was present in 10 cases (23.8%). Two duodenal ulcer cases were Helicobacter pylori positive
(100%) and one gastric ulcer case was Helicobacter pylori positive (33.3%).
Conclusion: The endoscopic biopsy not only permits exact diagnosis of specific entity but also provide an opportunity to see H. pylori status and plans for specific medical or surgical therapy. However histopathological study detects mucosal lesions at an early stage especially atrophy, intestinal metaplasia and dysplasia as to prevent progress of these lesions to invasive cancer.
Keywords: Endoscopic biopsies, Upper gastrointestinal tract, Squamous cell carcinoma, Adenocarcinoma
Full Text:
INTRODUCTION
Upper gastrointestinal tract is a common site for neoplasms, especially malignant tumours. Worldwide, gastric adenocarcinoma is the second most common cancer, and carcinoma esophagus is the sixth leading cause of death.1,2 In India, according to the National Cancer Registry, esophageal and gastric cancers are the most common cancers found in men, while esophageal cancer ranks third among women after carcinoma of breast and cervix.3 Squamous cell carcinoma of esophagus is usually seen in the middle third. Squamous cell carcinomas of the oesophagus emerges mainly through a sequence of chronic-esophagitis, progressive dysplasia, carcinoma in situ, finally invasive carcinoma (Munoz et al. 1982). In the stomach, the great concern is to pick the early gastric lesions on histopathology of the gastric mucosal biopsies (early gastric cancer) that does not extend beyond the mucosa or submucosa even though there may be lymph node metastasis in contrast to the invasive gastric carcinoma which extend beyond muscularis propia where the prognosis is poor.4 Endoscopy provides numerous small mucosal biopsies to diagnose and monitor the course of a variety of gastrointestinal tumours. It allows for biopsy under direct vision and opportunity arises from improved correlation of histologic features with gross features of the disease in both early and advanced phases. Thus, the role of upper gastrointestinal mucosal biopsies for the histopathological identification of the earlier stages of various gastrointestinal tumours by endoscopy allows an early therapeutic decisions without unnecessary delay. Helicobacter pylori (H. pylori) are found more frequently within the mucus layer and in the gastric pits, on electron microscopy the organisms are adherent to the surface epithelial cells. The organisms are often visible on Hematoxylin and Eosin staining, though are more easily seen with a Giemsa stain, which is useful in detecting small numbers. Warthin Starry Stain demonstrates H. pylori well but is expensive and seldom used in routine work.5,6 It has been confirmed that the development of gastric cancer spans several decades sequentially starting from the H. pylori infection, development of chronic active gastritis to development of glandular atrophy and intestinal metaplasia in a subset of patients.7 The Sydney system for the classification of gastritis emphasizes the importance of topographical, morphological and etiological information.8 This system was revised at the Houston Gastritis Workshop held in 1994. The histological severity of Helicobacter pylori density, inflammation, activity, atrophy and intestinal metaplasia were graded according to the updated Sydney system.9
AIMS AND OBJECTIVES
• Study the histopathology of endoscopic biopsies of upper gastrointestinal tract.
• Study the correlation between various histopathological lesions of upper gastrointestinal tract with age, sex and clinical presentation.
• Study the Helicobacter pylori density in Gastritis, Gastric ulcers and Duodenal ulcers.
MATERIALS AND METHODS
The study was conducted in the Postgraduate Department of Pathology, Government Medical College, Srinagar on 132 upper gastrointestinal endoscopic biopsies performed at Shri Maharaja Hari Singh (SMHS) and its associated hospitals during two and half year period from January 2011 to June 2013. Brief clinical data was noted from the case records, which included the age, sex and presenting symptoms of the patients. Biopsies obtained were oriented, fixed in 10% neutral formaldehyde and routinely processed. Approximately 5μm thick serial sections were cut and stained with hematoxylin and eosin (H and E). In addition, a special stain Giemsa was performed to confirm the presence of H. pylori. For detection of H. pylori induced lesions, the surface epithelium and foveolarlumen were searched for curved bacilli in H and E. Giemsa stain was used to facilitate their recognition. Organisms were searched under oil immersion (100X). The sections were examined for various histopathological features related togastritis like chronic and mixed inflammatory infiltrates, neutrophilic activity, intestinal metaplasia, atrophy, and presence of H. pylori. The sections were also examined for the presence of malignant changes. The findings were then correlated with the age, sex, and clinical presentation.
Inclusion Criteria
• All upper gastrointestinal endoscopic biopsies performed at SMHS and its associated hospitals and referred to Postgraduate Department of Pathology, Government Medical College, Srinagar.
• Lesions present in oesophagus, stomach and up to second part of duodenum (D2).
Exclusion Criteria
• Patients presenting with lesions in the oral cavity and pharynx.
• Patients presenting with lesions beyond the second part of duodenum (D2).
OBSERVATIONS AND RESULTS
The present study included 132 upper GIT endoscopic biopsies between the period January 2011 to June 2013. The following results were observed
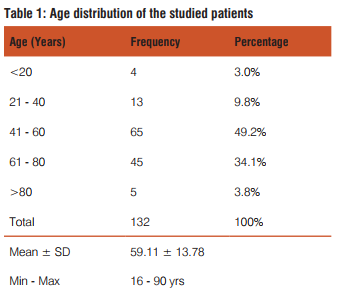

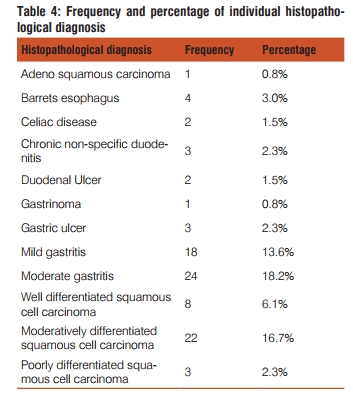

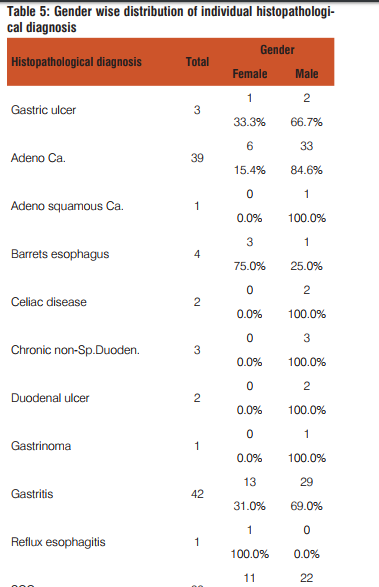
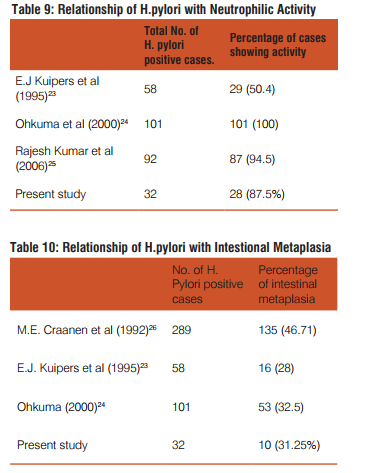
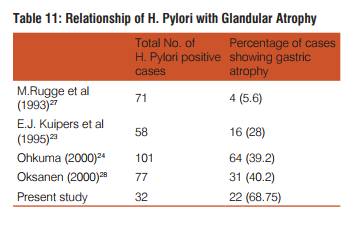
Dysphagia was common complaint (100%) in patients of squamous cell carcinoma, epigastric pain in adenocarcinoma patients(35.9%), dyspepsia was common in celiac disease(100%),duodenal ulcer(100%) gastritis(71.4%) patients. Out of total 42 cases of gastritis H. pylori was present in 32(76.2%) with grade 1 in 20(47.6%) and grade 2 in 12(28.6%). H. pylori was absent in 10(23.8%) gastritis patients. Out of 3 gastric ulcer cases, one (33.3%) was H. pylori present and out of 2 duodenal ulcer patients 2(100%) were H. pylori positive. Out of 32 H. pylori positive gastritis cases, 3 cases (9.4%) have chronic inflammatory infiltrate and 29 cases (90.6%) have mixed inflammatory infiltrate. While as 10(100%) H. pylori negative gastritis cases have chronic inflammatory infiltrate. Of total 32 H. pylori positive chronic gastritis, 28 cases (87.5%) show activity while out of 10 cases of H. pylori gastritis cases only 2 cases (20%) shows activity. Out of total 32 H. pylori positive gastritis, atrophy was present in 22 cases (68.75%) with mild atrophy in 20 cases (62.5%) and moderate in 2 cases (6.3%) and out of total 10 H. Pylori negative cases, 4 cases (40%) shows mild atrophy. Out of total 32 H. pylori positive gastritis cases 10 cases(31.2%) shows intestinal metaplasia with 9 cases (28.1%) of mild and 1 case (3.1%) of moderate intestinal metaplasia and out of total 10 H. pylori negative cases 4 cases (40%) shows mild intestinal metaplasia.
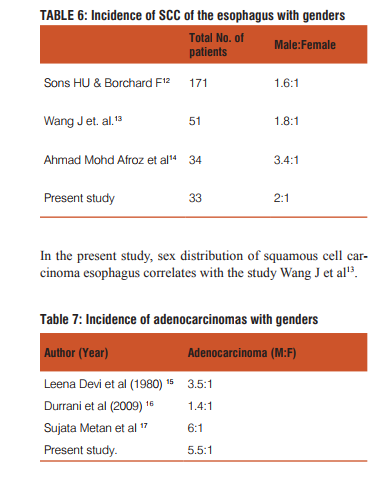
PHOTO MICROGRAPHS
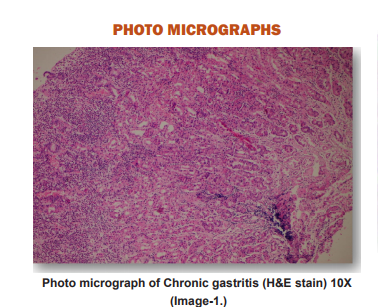
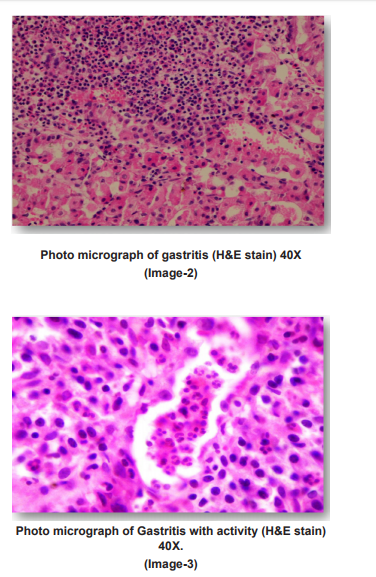
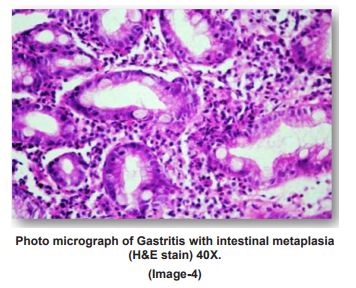

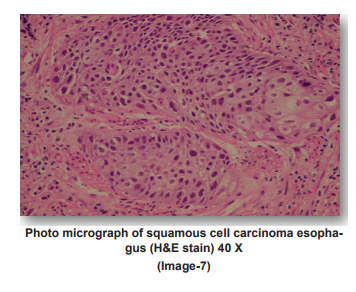

DISCUSSION
In the present study, a total number of 132 endoscopic biopsies from the upper gastrointestinal tract were received in the Post graduate Department of Pathology, at Government Medical College Srinagar. The present study was carried out for a period of 30 months, from 1st January 2011 to 30thJune 2013. The study included 37 esophageal biopsies, 13 gastroesophageal junction biopsies, 75 stomach biopsies and 7 duodenal biopsies.
Sex distribution of all cases:
Of the 132 patients with upper gastrointestinal tract endoscopic biopsies, 26.5% were females and 73.5% were males. This was also proved by another study done by Shennak MM et al10.The male: female ratio was 2.8: 1. This gender ratio favoring males could be reflective of the fact that males are exposed to more risk factors than females and gastrointestinal malignancies are more common in males according to JC Paymaster et al11 or due to large number of male patients attending outpatient department of the hospital as compared to female patients.
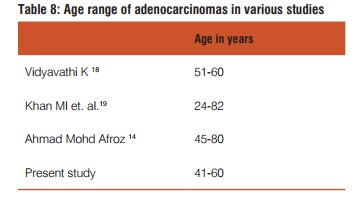
Our incidence of gender ratio of adenocarcinomas matches with that of Sujata Metan et al17.
Chief complaints of all cases:
In the present study the most common complaints were dyspepsia (37.9%), dysphagia (30.3%) followed by epigastric pain (18.2%), hematemesis (6.1%), recurrent vomiting (4.5%) post prandial distension (3%). Dysphagia was common symptom in patients of SCC (100%), same was reported by Kumar MK (1973)20, Gadour et al (2004)21 Epigastric pain in patients of adenocarcinoma (35.9%), followed by dysphagia (17.9%), dyspepsia (15.4%), recurrent vomiting (15.4%). Similar symptoms were reported by Gadour et al (2004)21, Sivagamani et al (1974).22
CONCLUSION
• Squamous cell carcinoma was a common malignancy histologically diagnosed from esophagus.
• Dysphagia was a common clinical presentation of squamous cell carcinoma cases.
• Adenocarcinoma was a common malignancy histologically diagnosed from gastroesophageal junction.
Epigastric pain was a common clinical presentation of adenocarcinoma cases.
• Histologically diagnosed gastritis was common from stomach and dyspepsia was a common clinical presentation of gastritis cases.
• Histologically diagnosed chronic non-specific duodenitis was common from duodenum and dyspepsia was a common clinical presentation.
• Helicobacter pylori was seen in maximum number of gastritis and duodenal ulcer cases.
• Majority of H. pylori gastritis cases show mixed inflammation, neutrophilic activity and glandular atrophy.
Thus the endoscopic biopsy not only permits exact diagnosis of specific entity but also provide an opportunity to see H. pylori status and plans for specific medical or surgical therapy. However histo pathological study detects gastric mucosal lesions at an early stage especially atrophy, intestinal metaplasia and dysplasia so as to prevent progress of these lesions to invasive cancer.
Conflict of interests
The author(s) have not declared any conflict of interests. Source of funding There is no source of funding.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed
References:
1. Zhang XF, Huang CM, Lu HS, Wu XY, Wang C, Guang GX et al. Surgical treatment and prognosis of gastric cancer in 2613 patients. World J Gastroenterol 2004; 10: 3405-3408.
2. EnzingerPC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349: 2241-52.
3. National Cancer Registry Programme. First All India Report 2001-2002. Vol. 1. Indian Council of Medical Research Bangalore, India. April 2004.
4. Evan’s D. MD, Craven JL, Murphy F and Clearly BK. Comparison of early gastric cancer in Britain and Japan. Gut 1978; 19: 1-9.
5. D.R. Saha. Studies on Helicobacter pylori: National Institute of Cholera and Enteric Diseases. Annual Report 2004-2005.
6. Singh V, Trikha B, Vaiphei K, Nain CK et al. Helicobacter pylori: Evidence forspouse-to-spouse transmission. Journal of Gastroenterol. Hepatol. 1999; 14: 519-522.
7. C.S Goodwin, JA Armstrong, BJ Marshall. Campylobacter pyloridis, gastritisand peptic ulceration. J Clinic Pathol 1986; 39: 353-365.
8. Kobayashi O, Eishi Y, Ohkusa T et al. Gastric mucosal density of Helicobacter pyloriestimated by real-time PCR compared with results of urea breath test and histologicalgrading. J Med Microbiol 2002; 51: 305-11.
9. Ozturk S, Serinsoz E, Kuzu I et al. The Sydney System in the assessment of gastritis:Inter-observer agreement. The Turkish Journal of Gastroenterology 2001; 12: 36-9.
10. Shennak MM, Tarawneh MS, Al-Sheik.Upper gastrointestinal diseases in symptomatic Jordanians: A prospectiveendoscopic study. Ann Saudi Med 1997; 17(4): 471-74.
11. Paymaster JC, Sanghvi LD, Ganghadaran P. Cancer of gastrointestinal tract in western India. Cancer 1968; 21: 279-87.
12. Sons HU, Borchard F. Cancer of the distal esophagus and cardia: incidence, tumorous infiltration, and metastatic spread. Ann Surg. 1986; 203: 188–195.
13. Wang J, Noffsinger A, Stemmermann G, Fenoglio-Preiser C. Esophageal squamous cell carcinomas arising in patients from a high-risk area of north China lack an association with EpsteinBarr Virus. Cancer Epidemiology Biomarkers and Prevention 1999 1999; 8(12): 1111-4.
14. Ahamed Mohammed Afroz, Bharathi Muniyappa. Histopathological study of neoplastic lesions of upper gastro intestinal tract-by endoscopic biopsy. Thesis submitted to Rajiv Gandhi University of Health Sciences, Karnataka, Bangalore, April2012.
15. Devi Leena KR and Suvarna N. Pattern of gastrointestinal tumours in north Kerala. Indian J of Cancer 1980; 17: 159-163.
16. Durrani AA, Yaqoob N, Abbasi S, SiddiqM, Moin S. Pattern of upper gastrointestinal malignancies in northern Punjab. Pak J Med Sci 2009; 25(2): 302-307.
17. Sujata Metan, P.V. Patil. Upper Gastrointestinal Tract Endoscopic Biopsies – A Histopathological Study. A One Year Cross Sectional Study. Thesis Submitted to KLE’S Dr. Prabhakar Kore Hospital, Belgaum, KLE University, Belgaum, Karnataka. 2011.
18. Vidyavathi K, Harendra Kumar ML, Lakshmana Kumar YC. Correlation of endoscopic brush cytology with biopsy in diagnosis of upper gastrointestinal neoplasms. Indian J Pathol Microbiol 2008; 51: 489-92.
19. Khan MI, Baqai MT, Bukhari M, Hashmi RI. Gastric Carcinoma: 5 Years survival after gastric surgery. 2005; Vol. 55; No. 4: pages 158-60.
20. Kumar MK and Ramachandran P. Carcinoma esophagus in north Kerala. The Indian J of Cancer 1973; Jun: 183-187.
21. Gadour MO and Ayoola EA. The frequency of upper gastrointestinal malignancy in Gizan. Saudi J Gastroenterol 2004; 10(1): 16-21.
22. Sivangamani K, Reddy B, Changal R. Carcinoma of the stomach – A study of 200 cases. The Indian J of Cancer 1974 Dec: 437- 443.
23. E.J. Kuipers, A. M. Uyterlinde, A.S. Pena, R. Roosendaal et al. Long-term sequelae of Helicobacter pylori gastritis: The Lancet 1995; 345: 1525-1528.
24. Ohkuma K, Okada M, Murayama H, Seo M, Maeda K, Kanda M, Okabe N. Association of Helicobacter pylori infection with atrophic gastritis and intestinal metaplasia. J Gastroenterol Hepatol. 2000 Oct; 15(10): 1105-12.
25. Rajesh Kumar, G. Bano, B. Kapoor, Sunil Sharma et al. Clinical Profile in H. pylori Positive Patients in Jammu: JK Science 2006; 8(3): 148-150.
26. M E Craanen, W Dekker, P Blok, J Ferwerda, G N J Tytgat. Intestinal metaplasia and Helicobacter pylori: an endoscopic bioptic study of the gastric antrum. Gut, 1992; 33: 16-20
27. M. Rugge, F.DI. Mario, M. Cassaro, R. Baffa et al. Pathology of the gastric antrum and body associated with Helicobacter pylori infection in non-ulcerous patients: is the bacterium a promoter of intestinal metaplasia? Histopathology 1993; 22: 9-15.
28. A. Oksanen, P Sipponen, R Karttunen, A Miettinen et al. Atrophic gastritis and Helicobacter pylori infection in outpatients referred for gastroscopy: Gut 2000; 46: 460-463.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License