IJCRR - 4(16), August, 2012
Pages: 134-145
Date of Publication: 28-Aug-2012
Print Article
Download XML Download PDF
ISOLATION OF EXTRACELLULAR FUNGAL PECTINOLYTIC ENZYMES AND EXTRACTION OF PECTIN USING KINNOW WASTE AS SUBSTRATE
Author: Yogesh Saini, Ramavtar Sharma, Mukesh Kumar, Sumer Singh, Pawan Kumar
Category: General Sciences
Abstract:Various types of fungal species have been reported to be employed for production of pectinases. As extracellular pectinases are easier to harvest and thus the scale up is cheaper and simpler. Thus in the present study, kinnow waste was used to generate extracellular fungal pectinases from natural sources along withextraction of pectin. Morphological examination of screened isolates revealed that Isolate#1 and Isolate#3 could be of Rhizopusgenus and Isolate#2 could of Aspergillus genus. Aspergillusoryzaevaroryzaewas used as standard fungi in the present. Quantitative estimation of total protein was made to know total enzymatic activities generated from isolates. It was done via SmF as well as SSF and pure pectin resulted in maximum activity among substrates, followed by peel powder and finally pomace. In SmF, among all isolates, Isolate #3 was found to produce maximum total protein and all other enzymatic activities(Total protein-8.69mg/ml/10min, Pectinase-763.82 nmoles/ml/60min, Polygalacturonase activity-99.83 nmoles/ml/10min andPL-1038.68nmoles/ml/15min). Extraction of pectin resulted in 20% yield from kinnow peel powder, 80% Ethanol was found to be suitable for extraction of pectin and 7.3% calcium pectate was obtained.
Keywords: Kinnowpeels; Polygalacturonase; Aspergillusoryzaevaroryzae; Pectinase; Extracellular fungal
Full Text:
INTRODUCTION
Kinnow is one of the most important citrus crops of northern India especially of Punjab, which produces about 0.180 MMT of kinnow, accounting over 50% of the national produce (Hindustan Times; May 7, 2011). Kinnow is highly relished as a fresh fruit owing to its rich organoleptic and thirst quenching properties and has a high appeal. Steps are already underway to harness the immense potential ofthis cropandtap the market, by attacking the major problem of bitterness in extracted kinnow juice. The attempts have been mainly based on curative approaches and of late, preventive approach is also being worked out. Processing and utilization of kinnow into various products eventually leads to generation of waste in form of peels and pomace. Kinnow waste is conventionally biotransformed anaerobically into humus, although many valuable byproducts can be produced from the rich waste. In other words wealth can be derived from this waste by value addition and products such as pectin, peel oil, pomace powder, dietary fibers and predominantly pectinases can be easily harnessed. Of these products, pectin and pectinases have a wide global market. Pectinases accounts for 10% of global industrial enzymes produced and their market is increasing day by day (Stutzenberger, 1992). Pectinases are the group of enzymes, which cause degradation of pectin that are chain molecules with a rhamnogalacturonan backbone, associated with other polymers and carbohydrates. These pectinases have wide applications in fruit juice industry and wine industry. In fruit juice industry, it is used for clarification, where reduction in viscosity is caused which ultimately leads to formation of clear juice. They increase the yield of juices by enzymatic liquefaction of pulps; these pectinases also helps in formation of pulpy products by macerating the organized tissue into suspension of intact cells. In wine industry pectinases are mainly used for decreasing astringency by solubilizing anthocyanins without leaching out procyadin polyphenols, and pectinases also increase pigmentation by extracting more anthocyanins(Tucker and Woods, 1991) Pectinases can be produced by both submerged and solid state fermentation (SSF). Submerged fermentation is cultivation of microorganisms on liquid broth. It requires high volumes of water, continuous agitation and generates lot of effluents. SSF incorporates microbial growth and product formation on or within particles of a solid substrate (Mudgett, 1986) under aerobic conditions, in the absence or near absence of free water, and does not generally require aseptic conditions for enzyme production. Many filamentous fungi like Aspergillusniger, Aspergillus. awamori, Penicilliumrestrictum, Trichodermaviride, Mucorpiriformis and Yarrowialipolytica.etc are used in both submerged as well as solid state fermentation for production of various industrially important products such as citric acid, ethanol etc. Fungi like Aspergillusniger, Aspergillusoryzae, Penicilliumexpansum, which are generally regarded as safe (GRAS) by United States Food and Drugs Administration (USFDA) are employed in food industry (Pariza and Foster, 1983).Some bacteria (Bacillus licheniformis, Aeromonascavi, Lactobacillus etc), yeasts like Saccharomyces, Candida and Actinomycetes like Streptomycetes are also used. Amongst these, the filamentous fungi are most commonly employed (Pandeyet al, 1999). Fungi can produce both intracellular as well as extracellular enzymes. All fungi are hetrotrophic, and rely on carbon compounds synthesized by other living organisms. Small molecules like mono disaccharides fatty acids and amino acids can easily pass through but for breaking down of larger complex compounds like pectin, fungi secrete extra cellular enzymes. It is well known that as compared to intracellular enzymes, the extra cellular enzymes are easier to be extracted. Intracellular enzymes require more time and costly chemicals for extraction (Hankin and Anagnostakis, 1975).Till date, Substrates used for solid-state fermentation are materials of plant origin like grains rice, corn, root, tubers, and legumes. Apart from these, pomace, mango peels, orange waste like peels and other fruit and vegetable industry waste are also being in much use (Smith and Aidoo, 1988) Kinnow waste also holds a promising substrate because of rich pectin content in peels. Sudhakar and Miani (1992) have reported 18.3% pectin in mandarin peels, which is quite close to the pectin content of apple pomace and is utilized for commercial extraction of pectin (Girdharilal et al, 1998). As Pectin is the ideal substrate for production of Pectinases; it was thought that attempts should be made to extract pectinase from kinnow waste after isolation of potential pectinolytic fungi from natural sources. Till now, no report isavailable on pectinase production using kinnow waste as substrate. Also, pectin itself can be extracted from kinnow waste (mainly from peels) as a commercially important by-product. Apple, lemon, orange, mango, tomato, beet, carrots etc are the commonly used sources for extraction of pectin. Of these, mango peels, apple pomace, lemon pulp and orange pulp are most commonly used. Pectin finds wide application in the manufacture of many fruit products like jams, jellies, marmalades, preservatives etc and thus are indispensable to the fruit juice industry. It is also used as a thickening agent for sauces, ketchups, flavored syrups and as a texturing agent in fruit flavored milk desserts (Girdharilalet al, 1998). In view of the above-mentioned points, the present investigation was undertaken and attempts were made to produce pectinases from the isolated fungi from natural sources and to extract pectin from kinnow Waste as substrate.
MATERIALS AND METHODS
Material procurement
Kinnow as natural source of pectinolytic fungi Rotten and fungus infected kinnow fruits were collected from different fruit shops/ vendors in Bikaner in the month of January 2012.
Kinnow peels and Kinnowpomace powder
Kinnow peels and Kinnowpomace, which is juice extracted kinnow fruit, were collected from different fruit shops/vendors in Bikaner and also from Plant Biotechnology Cente, Swami Keshwanand Rajasthan Agariculture University ,Bikaner , where another project on kinnow fruit was going on during the course of present study. Kinnow peel powder and pomace powder was made by followingprocedure - Peels and pomace were first washed many a times with water to remove all adhering substances. Small pieces of peels were made using knife and these were then dried in tray drier at 500C for 24 hr with intermittent shaking. Similarly washed pomace was well spread uniformly and dried. The dried kinnow peels and pomace were then made to powder using a mechanical grinder (Philips India limited, Kolkata).
Aspergillusoryzaevaroryzae (as standard)
Freeze dried culture of Aspergillusoryzaevaroryzae (MTCC 3567) were collected from Microbial Type Culture Collection and Gene Bank(MTCC), Institute of Microbial Technology (IMTECH), Chandigarh. The culture was activated according to the following method mentioned as by suppliers: 1. Firstly, a mark was made on the ampoule near the middle of cotton wool with a sharp file. The surface around the mark was then disinfected with 100% alcohol. 2. The ampoule was then wrapped with thick cotton wool and marked area was broken, cotton plug was then carefully removed and 0.4ml sterile water was added to make a suspension. 3. The suspension was allowed to stand for 20 minutes, after 20 min, few drops of suspension were streaked onto Medium 117 (Annexure I) in number of petri plates and slants. 4. Rest of the suspension was transferred to 5 ml of Medium 117(broth) in a test tube. 5. The plates were then incubated in BOD at 250C for 5 days. 6. After the growth of fungi, fungal culture was maintained by subculturing the fungi on the same medium throughout the experimental period. Chemicals, media and standard enzyme All the chemicals used in the present investigation were of Analytical grade (AR grade). Dgalacturonic acid and Disodium hydrogen arsenate were procured from Sigma, Aldrich Corp, MO, USA. Polygalacturonic acid, Neocuproine, pectin, Congo red dye was procured from Himedia Laboratories Limited, Mumbai. Standard enzyme- Pectinase was procured from Himedia Laboratories Limited, Mumbai. All the experiments for isolation, screening and pectinolytic enzyme production were done under sterile conditions and adequate safety measures were undertaken (Annexure IX)
Isolation of Pectinolytic fungi
Rotten and fungus infected kinnows were swabbed in 0.8% saline in an autoclaved stomacher bags. Dilutions upto10-8 was made and pour plating of higher dilutions namely 10-5, 10-6, 10-7, and 10-8 was done using melted CzapekAgar (Annexure II). The above dilutions were plated in duplicates. The plates were then incubated at 300C for 12 days. The colonies thus isolated were then subcultured3- 4 times on Czapek agar till active growth of isolates were obtained, and thereafter maintained on the same media for further experimental work.
Screening of isolates having pectinolytic activity
Qualitative test for screening of isolates having pectinolytic activity For screening purpose, Czapek agar having1% pure pectin was used as sole carbon source and Congo red (@150mg/l) was also added in medium, so as to clearly visualize the formed clear zones. Simultaneously, control plate was set having Congo red but without pectin or any other carbon source. Following methodology was adopted for screening of potential isolates -Using a flamed and cooled cork borer, one disc of fungal hyphae from leading edge of actively growing colonies was cut on petri plate. With a flamed and cooled transfer needle, discs were then transferred to Czapek agar media having pectin as sole carbon source. Plates were then incubated at 300C for 12 days. Selection was done on the basis of formation of clear zones and the corresponding diameters were noted during that span of time. Morphological examination of isolates was done using Image Analyser having compound microscope. Quantitative estimation of pectinolytic activity of screenedpectinolytic isolates Quantitative estimation of pectinolytic activity screened isolates was done on submerged as well as solid state fermentation. Submerged Fermentation In order to undertake submerged fermentation firstly pure pectin was used as substrate, subsequently it was replaced by kinnow peel powder and then finally by kinnowpomace powder. For each isolate, 2 flasks were prepared; one was treated as test flask and the other as control flask. In every sterile flask, 1 disc of respective fungal isolate was added and the flasks were properly plugged. The flasks were then incubated in incubator shaker for 12 days at 300C at 250 rpm. Aliquots were withdrawn every 0, 3, 6, 9, 12 day for carrying out assays namely: Total protein, Pectinase assay, Polygalacturonase assay and Pectin Lyase assay according to methods explained in section 3.5. Experiments were also conducted to know the effect of number of fungal discs (1, 2 and 3) as well as amount of pectin 1% and 2% on total protein using the above methodology. Solid State Fermentation Solid State Fermentation was carried out in sterile250ml flasks. Experiments were performed using only kinnow peel powder as sole carbon source. For every isolate and standard fungus, experiments were set up using 5 sterile flasks. Aliquots were drawn after adequate dilution (10 times) on every 0, 3rd, 6th, 9th and 12thday.
Solid State Fermentation was carried as follows:
15g of substrate was taken and 8ml of sterile water was added. One disc of respective fungal hyphae was mixed to 5ml of sterile water and a suspension was made. From this suspension, 1ml was withdrawn and inoculated into each of the 5 flasks. The flasks were then incubated at 300C for respective period of time. For proper aeration, flasks were intermittently shaken. In order to estimate the Total Protein, Pectinase, Polygalacturonase and Pectin Lyase, 10ml ofSterile water was added and then properly mixed; the mixture thus obtained was gently shaken and was filtered using coarse filter paper.The obtained filtrate was then centrifuged and resulting supernatant was used for conducting the assays.
Assays
In order to quantify total protein and pectinolytic enzymes produced via submerged and solid state fermentation, following experiments were conducted: Total protein content (BIURET METHOD) 5mg albumin/ml was used as protein standard Reagents used: Biuret reagent (Annexure III) Firstly standard curve with different concentrations of bovine albuminserum (BSA) namely-100l, 150l, 200l, 250l 300l was made. For analysis of total protein, 3ml of biuret reagent was added to 2ml of test protein solution in a sterile test tube and the mixture was properly mixed. The tubes were then warmed at 370C for 10 min with shaking and finally the tubes were cooled and absorbance was noted at 540nm.
Pectinase assay
WBC Home manual/protocol index methodology was followed to estimate the pectinase activity. Reagents used:
I) 0.1 M Phosphate buffer pH 5.0
ii) Color reagent A (Annexure IV)
Iii) Color reagent B (Annexure V)
IV) D-galacturonic acid-1mg/ml
V) 0.5%Polygalacturonic acid substrate (Annexure
VI)
1. In one test tube 6ml of substrate and 1ml of buffer was added and thenproperly mixed (reagent blank).
2. In second test tube 6ml substrate and 1ml of enzyme solution 0.1mg/mlwas added (test sample).
3. In third test tube 6ml buffer and 1ml enzyme sample at 1mg/ml (sampleblank) was taken.
4. For standard D- galacturonic acid, different concentration 0, 20, 40, 60, 80, 100, 120 ?g of D-galacturonic acid was used.
5. The above three reaction tubes along with standard tubes were incubated at 370C water bath with shaking for 60min±1min. After incubation the tubes were immediately placed into ice water to stop reaction.100µl aliquot was drawn from each tube and was pipetted into another set of tubes also placed on ice water.
6. To each reaction tube and standard tube. 2ml color reagent A and 2ml color reagent B was added, and the mixture was mixed properly by inversion. The tubes were then placed into boiling water bath for 13min±1min. The tubes were then cooled and then 2ml water was added. Again proper mixing was done by inversion. The absorbance was noted at 450nm using water blank and disposable cuvettes were used throughout the assay owing to sticking of formed orange color in cuvettes.
Polygalacturonase (PG)
PG assay was carried by method as described Baldwin and Pressey(1989).
1. In a test tube, 2ml of 0.1M Sodium acetate, .025ml of 0.15 M Sodiumchloride, 0.5ml of 1% polygalacturonic acid and .05ml of enzymesolution was taken. Test tubes were then incubated at 370C for 15 minin water bath with shaking.
2. The above solution was then analyzed for reducing groups following theArsenomolybdate method given by Nelson Somogyi (1952). Reagents usedAlkaline copper tartarate (Annexure VII) Arsenomolybdate reagent (Annexure VII)
i) Aliquots of .1 or .2 ml of above solution was pipetted out in test tube.
ii)0.2,0.4,0.6,0.8,1.0 ml of standard solution (100?g/ml glucosesolution) were taken into series of tubes.
iii) The volume in both sample and standard tubes was made to 2ml with distilled water
IV) 2ml distilled water was pipetted out in separate test tubes to set up ablank. v) 1ml of alkaline copper tartarate reagent was added to each tube
vi) The tubes were then placed in boiling water bath for 10 minutes andthen cooled; and 1ml of arsenomolybdolic reagent was added to alltest tubes taking full precautions
vii) The volume in each tube was then made to 10ml with water
viii) The absorbance of blue color was then determined at 620 nm after10 min.
PectinLyase Assay
Pectin lyase was assayed by measuring the increase in absorbance at 235nm according to method described by Albersheim and Killias (1962). In a properly washed and cleaned test tube 0.2ml of 0.1M Sodium acetate (pH 5.5), 25ml of 0.12M sodium chloride, 0.5ml of 1% pectin (pH 5.5) and 0.05ml of enzyme solution was pippeted out and mixed. The tubes were then incubated at 370C for 15 min in water bath with shaking. 5ml of water was then added to the above reaction mixture and absorbance was then determined at 235nm.
Extraction and estimation of pectin
Pectin was extracted by method given by Rao and Maini, (1999) as shown in Figure 2.1. 15 g of peel powder was weighed and was taken in 150ml flask; to it 30ml of dilute acid .05N Hydrochloric acid was added. Extraction was done by boiling the above mixture at 1000C for 60 min. The marc was then separated from the extract after filtration. The extraction was performed2 Times. Filtrates obtained were then combined and then cooled. Two volumes of absolute alcohol were added to precipitate pectin. Pectin was also precipitated using different strengths of alcohol namely 90%, 80%, 70%, 60%, and 50%. Yield of pectin was calculated by the following formula:
Amount of pectin obtained x 100
Yield of pectin = --------------------------------------------
Amount of peel powder
Estimation of pectin (Ranganna, 2000)
The procedure followed was as follows: 1.200mg of dried pectin was weighed into a 1-litre beaker and it was wetted with 2 or 3 ml of 100% alcohol. 2.400ml of water was added with stirring to it. 3. The solution was then boiled and then cooled immediately. 4. Then it was transferred into a 500ml volumetric flask and volume was made up. 5. 200ml aliquot was pipetted out each into two 1l beakers. 6. 250ml water was added and solution was neutralized with 1N Sodium hydroxide using phenolphthalein as indicator Afterwards, 10ml of 1N Sodium hydroxide was pipette out in excess with constant stirring and was allowed to stand overnight. 7. Then next day 50ml of 1N Acetic acid was added and after 5minutes25ml of 1N calcium chloride was added with stirring. 8. Solution was allowed to stand for 1 hour, and was then boiled for 2 min. 9. The solution was filtered using previously prepared filter paper (filter paper was made wet in hot water, dried in oven at 1020C for 2 hours, cooled in a dessicator and weighed in a covered dish) precipitate obtained was washed thoroughly with boiling water. 10. In an originally weighed dish, the filter paper containing calcium pectat was transferred and was dried overnight at 1000C. 11. Weighing dish was cooled in dessicator and then it was finally weighed. % Calcium pectate was calculated using following formula:
Wt of calcium pectate x 500 x 100
% Calcium Pectate = -----------------------------------------------
Ml of filtrate taken x wt of
Sample taken for estimation
RESULT AND DISCUSSION
The present investigation was undertaken in order to harness the valuable products namely fungal pectinases as well as pectin from waste obtained from processing/ utilization of kinnow fruits. As it is well reported that certain sporulating strains like Aspergillusniger, Aspergillusoryzae, Rhizopusoryzaeetc have pectinolytic activity, the purpose was to screen similar isolates from nature and employee them for the production of pectinolytic enzymes. Only extracellular pectinases were targeted because in comparison tointracellular pectinases, extracellular are easier to harvest and scaling up work can be more easily attempted. Furthermore, as the isolated strains were to thrive only on pectin, pectin content of kinnow fruit was determined. The results of experiments performed to quantify enzymatic activity as well as amount of pectin are being systematically represented below.
Isolation of pectinolytic fungi
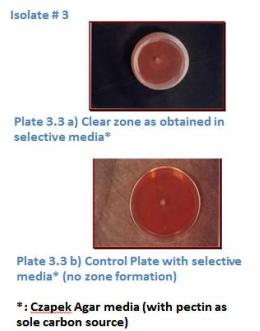
The collected rotten and fungus infected fruits were used as source of natural pectinolytic fungi and such fruits were collected in the month of January 2012. From the collected samples, eight potential fungi were screened for pectinolytic activity on selective media (Czapek media having pure pectin as the only carbon source) in duplicates, by taking higher dilutions from 10-5 to10-8 . Plates having Czapek media without any carbon source were treated as control, which showed no sign of growth. Based on visual observance, only three isolates gave clear zones of diameter 2.3mm to 4.1mm as shown in Plate 4.1 -4.3. In the first isolate, clear zone of diameter 2.9mm was observed on 4th day of its incubation at 300C, for the second isolate clear zone of diameter 2.2mm was observed on 6th day of its incubation, and for isolate#3 zone of 4.1mm was observed on 4th day; thereafter excessive growth was seen in each plate thus covering entire plate by mycelia. On the basis of literature review, Aspergillusoryzaevaroryzae and Rhizopusoryzae were treated as standard. Freeze dried culture of R. oryzae failed in being activated even after performing repeated trials. Thus onlyAspergillusoryzaevaroryzae was treated asstandard in the present study.
Morphological examination of the screened isolates
Morphological examination of screened isolates was performed using Image Analyser with compound microscope. Isolate # 1 and Isolate # 3 showed clear aerial hyphae, and black colored spores were also found by macroscopicalexamination microscopic examination showed presence of sporangiospores in sporangia at the tip of fertile hyphae, the sporangiophore. Sporangiophoreswere arising from the nodeand sporangia were present at the end of sporangiophores. The swollen tip of sporangiophorecolumella, projecting into sporangia, was also seen. Rhizoid like structures were also found arising from node as shown in Plate .4 (a and b) and in Plate 3.6 (a and b). As these characters belong to Rhizopus genus from this observation it can be inferred that Isolate # 1 and Isolate # 3 belongtoRhizopus genus. Isolate #2

showed definite zones of growth and mycelia were greenish. Spore bearing heads were seen as large and globular and were tightly packed.Chains of conidia were also seen. Conidia weregreenish in color. From these observations, it can beinferred that Isolate#2 might be belonging to Aspergilus genus (Plate3.5 a and b).
ANNEXURE
ANNEXURE
I Medium 117 Composition of Czapek yeast extract agar (CYA) Czapekcocncentrate 10.0ml K2HPO4 1.0g Yeast extract 5.0g Sucrose 30.0g Agar 15.0g Disttiled water 1.0L Czapek Concentrate
NaNO3 30.0g KCl 5.0g MgSO4.7H2O 5.0g FeSO4.7H2O 0.1g Distilled water 100.0ml
ANNEXURE II
Czapek agar Stock solution A50.0ml Stock solution C50.0ml Sucrose 30.0g Zinc solution 1ml Copper solution 1.0ml Agar 20.0g Distilled water 1 litre Stock solution A NaNO3 4.0g KCl 1.0g MgSO4.7H2O 20.0mg Distilled water 100.0ml Zinc solution ZnSO4.7H20 1.0g Distilled water 100.0ml Stock solution C K2HPO4 2.0g Distilled water 100.0ml Copper solution CuSO4.5H2O 0 .5g Distilled water 100.0 ml
ANNEXURE III
Biuret reagent Copper sulphate 3.0g Sodium potassium tartarate 9.0g (in 500ml of 0.2M/L Sodium hydroxide) Sodium iodide 5.0g Make up to 1L with 0.2mol/litre Sodium hydroxide
ANNEXURE IV
Color reagent A Sodium carbonate 40.0g (in 600ml water) Glycine 16.0g Copper sulphatepentahydrate Make up to 1L with Color reagent B Neocuprine-HCl 0 .450g
ANNEXURE V
1.2g Make up to 1 L with water and store at 40C in a brown bottle
ANNEXURE VI
.5% Polygalacturonic acid substrate Heated 500ml Phosphate buffer on hot plate. While heating slowly 2.5g polygalacturonic acid is slowly added. Heated and stirred until dissolved, cooled and stored at 40C.
ANNEXURE VII
Alkaline Copper tartarate (A) Anhydrous sodium carbonate 2.5g Sodium bicarbonate 2.0g Potassium sodium tartarate 2.5g Anhydrous sodium sulphate 20.0g Dissolved in 80 ml water and make up to 100 ml (B) Copper sulphate 15g. Dissolved in small amount of water added 1 drop of Sulphuric acid and make up to 100ml. Mix 4mlof B and 96 ml of A.
ANNEXURE VIII
Arsenomolybdate reagent Ammonium molybdate 2.5g Sulphuric acid 2.5ml Disodium hydrogen arsenate 0.3g(dissolved in 25ml water) Mixed well and incubated at 370C for 24 to 48 hours
ANNEXURE IX
Biosafety precaution while performing experiments
1. Hand gloves and mouth covers were worn while performing all fungal experiments in sporulating rooms.
2. Sporulating room was properly fumigated before performing experiments.
3. Discarded the fungi by properly autoclaving the flasks after performing experiments. Precautions taken during Nelson Somogyi method
1. Mouth covers and hand gloves were worn while handling dihydrogensodium arsenate, as it is very carcinogenic.
2. Mouth pippeting was completely avoide
ACKNOWLEDGEMENT
I thank the almighty whose blessings have enabled me to accomplish my dissertation work successfully. It is my pride and privilege to express my sincere thanks and deep sense of gratitude to Dr. Ramavtarsharma, Associate Professor Plant Biotechnology Centre, Swami keshwanad rajasthan agriculture university, Bikaner for her valuable advice, splendid supervision and constant patience through which this work was able to take the shape in which it has been presented. It was her valuable discussions and endless endeavors through which I have gained a lot. Her constant encouragement and confidence-imbibing attitude has always been a moral support for me.
References:
1. Aidoo, K.E.; Hendry, R.and Wood, B.J.B. 1982. Solid state Fermentation.Adv. Appl. Microbiol., 28, 201-237
2. Albersheim, P. and Killias, U.1962.Studies relating to the purification and properties of Pectin Transeliminase.ArchBiochemBiophys., 97, 107.
3. Alkorta,I.; Garbisu,C.; llama, M.J. and Serra, J.L.1998.Industrial applications of pectic enzymes: a review. Process Biochem., 33, 21-28.
4. Baldwin, E.A. And Pressey, R. 1989. Pectic enzymes in Pectolyase. Plantphysiol., 90, 191- 196.
5. Baracet, M.C.; Vanetti M, C.D.; Araujo, E.F.And Silva, D.O.1991. Growth conditions of PectinolyticAspergillusfumigatusfor degumming of natural fibers.Biotechnol.Lett., 13, 693-696.
6. Baron, A.; Rombouts, F.M.; Drilleau, J.F. and Pilnik, W.1980.Purification et proprietes de la Pectinesteraseproduite par Aspergillusniger. Lebensm, Wiss Technol.; 13, 330-333.
7. Beltman, H. And Pilnik, W. 1971. Die KramerscheScherpressealsLaboratoriumsPressvorrichtung und Ergebnisse von VersuchenmitAepfeln. Confructa .;16(1), 4-9.
8. Berovic, M. and Ostroversnik, H.1997.Production ofAspergillusnigerPectolytic enzymes by Solid State Bioprocessing of Apple Pomace.J. Biotechnol., 53, 47-53.
9. Braddock, R.J. And Kesterson, J.W.1979. Use of enzymes in Citrusprocessing. Food Technol., 33(11); 78.
10. Braddock, R.J.1983.Utilization of citrus juice vesicle and pelfiber.Food Technol. ; 37:85
11. Ceci, L. and Loranzo. J. 1998. Determination of enzymatic activities of commercial pectinases for the clarification of apple juice.Food Chem., 61, 237-241.
12. Charley, V.L.S. 1969. Some advances in food processing using pecticand other enzymes. Chem.Ind., 635-641.
13. Chesson, A. and R.C. Codner.1978.The maceration of vegetable tissue by a strain of Bacillus subtilis .J. Appl. Bacteriol., 44, 347- 364.
14. Cook, P.E. 1994.Fermented Foods as Biotechnological resources.FoodRes.Int., 27, 309-316.
15. GirdhariLal; Siddappa, G.S and Tandon G.L.1998. Manufacture ofpectin. Preservation of fruits and vegetables, Indian Council of Agriculture Research, New Delhi.., 265-272
16. Hang, Y. D. 1979. Production of Single Cell Protein from Food Processing Waste. Food Processing Management, AVI, Westport, Conn., 442-455.
17. Hang, Y.D. Luh.; B.S. and Woodams, E.E. 1994.Microbial production of citric acid by Solid State Fermentation of Kiwi fruit Peel.J. Food Sci., 52, 226-227.
18. Hankin, L. and Anagnostaksis, S.L. 1975. The use of solid media for detection of enzyme production by fungi. Mycology., 67, 597-607.
19. Houdenhoven, V., F. E. A. 1975. Studies on Pectin Lyase.Ph.D. Thesis.AgriculturalUniversity.Wageningen. Netherlands.
20. Hours, R.A.; Voget, C.E. and Ertola, R.J. 1988. Apple Pomace as Raw Material for Pectinase Production in Solid State Fermentation. BiolWastes., 23, 221-228.
21. Ishii, S.andYokotsuka, T.1972. Clarification of fruit juice by Pectin Transeliminase. Agric. Food Chem., 20, 787-791.
22. Ishii, S.andYokotsuka, T.1973.Susceptibility of fruit juice to enzymatic clarification by Pectin lyase and its relation to Pectin in fruit juice. J.Agric.Food Chem., 21, 269-272.
23. Kawano,C.Y.;Chellegatti,M.A.D.S.C.;Said,S.a ndFonsec,M.J.V.1999.Co mparative study of intracellular and extra cellular pectinases produced by Penicilliumfrequestan. Biotechnol. Appl.Biochem.,29, 133-140.
24. Mudgett, A.E.1986. Solid state fermentations in A. L. Demain and N. A. Solomon, eds. Manual of Industrial Microbiology and Biotechnology, American Society for Microbiology Washington, D.C., 66-83.
25. Pandey, A.1992.Recent progress developments in solid state Fermentation. Process Biochem., 27, 109-117.
26. Pandey, A.; Selvakumar, P.; Soccoi, C.R. And Nigam Poonam.2002.Solid State Fermentation for the Production of Industrial
27. enzymes.http://tejas.serc.iisc.ernet.in/~currsci/j uly10/articles23.html
28. Pariza, M.W. and Foster E.M.1983. Determining the safety of enzymes used in food industry. J. Food Prot., 46, 453-458.
29. Pastore, Profa. Dra.Glaucia Mari2001. Use of enzymesinFoodIndustry.ANBio - AssociacaoNacional de Biosseguranca.
30. Pilnik, W. and Voragen, A.G.J. 1993. Pectic enzymes in Fruit and VegetableJuiceManufacture.Enzymes in FoodProcessing,Academic Press Limited, London,Third Edition., 363-392.
31. Premi, B.R: Lal, G and Joshi, V.K.1994. Distribution pattern of bittering principles in kinnowfruit.J.FoodSci.Technol., 31, 2, 140- 141.
32. Garson, C.E. and Hours, R.A.1992. Citrus waste- An alternative Substrate for Pectinase Production in solid state cultures. BioresourceTechnol., 39, 93-95.
33. Raimbault, M.1998.General and Microbiological Aspects of SolidSubstrateFermentation.Electronic Journal Of Biotechnology., 1,3.
34. Ranganna.S.2000. Pectin. Handbook of Analysis and Quality Control for fruit and vegetable products, Tata McGraw Hill Publication, New Delhi, Second Edition., 40- 42.
35. Rao, S.D.V. and Maini, S.B. 1999.Manufacture of Pectins from Mango Peels. Beverage and Food world., 17-17-18.
36. Rombouts, F. M. andPilnik, W. 1980. Pectic Enzymes. Economic Microbiology (A. H. Rose, ed.), Vol.5, Academic Press, London.,227-282.
37. Sandhu, K.S.; B.S.; Shukla, F.C. 1985. Physicochemical changes during storage of kinnow mandarin oranges and pineapples juice concentrates. J. Food Sci. and Technol., 22, 342-345.
38. Sanzo, A.V.; Hasan, S.D.M.; Costa, J.A.V.andBertolin, T.E. 2001. Enhanced glucoamylaseproduction in semi-continuous solid-state fermentation of Aspergillusniger NRRL 3122. CienciaandEngenharia., 10, 59-62.
39. Singh, S.A.; Plattner, H. and Diekmann, H.1999a.Exopolygalacturonaselyase from thermophilicBacillus sp. Enzyme Microb Technol., 25,420-424.
40. Smith, J.E. and Aidoo, K.E. Growth of fungi on Solid Substrates. Physiology of Industrial Fungi, Blackwell, Oxford, England., 249-269.
41. Stutzenberger F. 1992.Pectinase Production. Encyclopedia of Microbiology (Lederberg J.ed-in-chief), Academy press, New York.3: 327-337.
42. Solis-Pereyra.S.; Favela-Torres, E.;GutierrezRojas,M.;Roussos,S.; Saucedo Castaneda, G. and Viniegra Gonzales, G.1996. Production of pectinases byAspergillusniger in solid state fermentation at high glucose concentrations. World.J.Microbiol. Biotechnol., 12, 275-260.
43. Somogyi, M. 1952.J biol., 200 257.
44. Thakur, B. R.; Singh, R. K and Handa, A.K. 1997.Chemistry and uses of pectin.Crit Rev Food SciNutr 37:47.
45. Tucker, G. A. and Woods, L. F. J. 1991. Enzymes in production of Beverages and Fruit juices. Enzymes in Food Processing, Blackie, New York.201-203.
46. Ueda, S.; and Fujino, Y. andLim, J.Y. 1982. Production and someproperties ofpectic enzymes from Aspergillusoryzae A3.J. Appl. Biochem., 4, 424-532.
47. Young,M. M.; Moriera, A. R. andTengerdy, R. P.1983. Principles of Solid state Fermentation in Smith J.E.; Berry, D. R.and Kristiansen, B, eds. Filamentous fungi Fungal Technology, Arnold, E. London. 117- 144. Hindustan Times, May7, 2011.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License