IJCRR - 5(1), January, 2013
Pages: 01-12
Print Article
Download XML Download PDF
HEXACHLOROBENZENE - SOURCES, REMEDIATION AND FUTURE PROSPECTS
Author: D.J. Mukesh Kumar, S. Dinesh Kumar, D. Kubendran, P.T. Kalaichelvan
Category: General Sciences
Abstract:Hexachlorobenzene (HCB) is one of the highly toxic and persistent compounds that are released unintentionally through various man-made chemicals. It is considered as a member of POPs (persistent organic pollutants) because it is persistent for long period of time in the environment and found to be hazardous for all living organisms. The present review focuses on the various sources of hexachlorobenzene present in the environment. Few Remediation technologies currently available for destruction of HCB from the environmental compartment such as soils, sediment and air were described in detail. Further, the transfer of HCB in to environment, their mode of action, toxicity, exposure and its health effects were also discussed.
Keywords: Hexachlorobenzene (HCB), Persistent Organic Pollutants (POPs), Remediation technologies
Full Text:
INTRODUCTION
Hexachlorobenzene (HCB) is a persistent nondegradable chlorinated hydrocarbon which was first introduced as fungicide in year 1945 for seed treatment. It is also found as an unintentional byproduct in the manufacture of chlorinated solvents such as carbon tetrachloride, perchlorethylene, trichloroethylene and pentachlorbenzene (Ritter et al., 1995). HCB is a white crystalline solid having 6 chlorine atoms and has low solubility in water (5 μg/l) at 25°C (Ramamoorthy and Ramamoorthy, 1997) but soluble in benzene, chloroform, ether, carbon disulfide, and boiling alcohol. It is quite volatile and can be expected to partition into the atmosphere as a result. It is found to be strongly resistant to breakdown and has a high partition coefficient (KOW=3.03-6.42), and is known to bioaccumulate in the fat of living organisms (Ritter et al., 1995).
The first reports on the toxicity of HCB was reported in 1950, describing liver damage, irritation of the respiratory system, and eye damage with the warning that the allowable concentration was 50 ppm, but that itself should be considered as dangerous (John Jarrell et al., 2000). However, hexachlorobenzene had come to public attention during 1954 -1959 when people of eastern turkey developed porphyria diseases, due to ingestion of HCB treated seed grains.
Although HCB is no longer used directly as a pesticide, it is currently formed as an unintentional by-product of several industrial sectors where both chlorine and carbon are present (Royal Haskoning, 2003). HCB is toxic, persistent, and liable to bioaccumulate and so is recognized as a POPs (Persistent Organic Pollutants). The HCB has a half-life from 2.7 to 6 years in water and in the atmosphere, and may have a half life of more than 6 years in soil (Mackay et al., 1992). According to the Environmental Protection Agency’s Toxic Chemical Release Inventory, in 1997 about 18 tonnes of hexachlorobenzene were reported to be accumulated per year as waste product (WHO 1997).
MODE OF TRANSFER TO THE ENVIRONMENT Due to its high persistivity and long range transport property, HCB is distributed throughout the environment. Hexachlorobenzene may enter the environment through air emissions, pesticide impurities, combustion products, municipal incinerators, volatilization and leaching from landfills, and emissions from combustion processes.
The total HCB emissions in atmosphere from 15 OSPARCOM countries in 1990 were 2851 kg/year (Berdowski et al., 1997). The world-wide emissions of HCB in the mid 1995 are estimated to be in the range of 12,000 to 92,000 kg/year (Bailey, 2001).
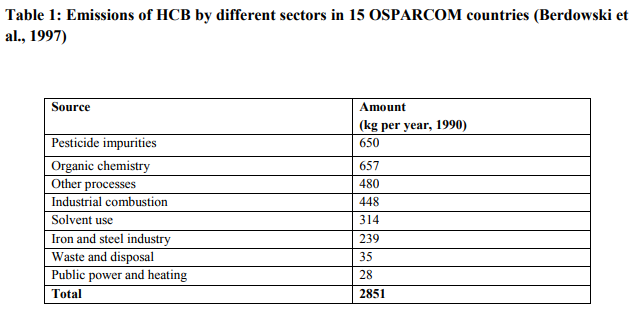
Soil is the major environmental media that has high impact over HCB exposure. Although the global volume of soil is considerably less than the volume of air, soil will contain a much greater mass of HCB than air. It is estimated that soil consists of higher HCB concentration than air and water. It has 88% of total HCB concentration (Fig.1). Thus transfer of HCB to the soil must seriously be taken into account (Tissier et al., 2005). The relationship between soil and air concentrations under equilibrium conditions is described by the soil/air partition coefficient (KSA). Reported log KSA values for HCB range from 7.27 (Meijer et al., 2003b) to 4.98 (Hippelein and McLachlan, 1998). Hexachlorobenzene is emitted onto soils by pesticides use and through waste disposal. Even though production of hexachlorobenzene as pesticide was discontinued in many countries, it is unintentionally released as a by-product in the manufacture of chlorinated solvents, chlorinated aromatics and chlorinated pesticides. Approximately 4130 tons/year of hexachlorobenzene were generated as a waste product in 1986 in USA and that nearly 77% of this was produced from the manufacture of three chlorinated solvents: carbon tetrachloride, trichloroethylene and tetrachloroethylene (WHO, 1997).
In case of air which has huge global volume, the partition coefficient of hexachlorobenzene will be high. But its concentration will be low in the air when compared to soil, because the release of HCB in this compartment is very low. Around the globe air comprises of 9% of total HCB concentrations ( Fig. 1). Industrial production of inorganic chemicals plays vital role in releasing HCB into the air. About 958 pounds were estimated to be present in the air during 1996, which comprises about 48% of total HCB concentration in the air. The production of silicone-based products, which includes antifoams, emulsions, hard coats, elastomers, adhesives, release coatings, and sealants, is reported as the source of HCB emissions from this source category. Production of pentachlorophenol emits Hexachlorobenzene in workplace air (Melnikova et al., 1975).
Next to air, the water and sediments consists of 1% and 2% of total HCB concentration in atmosphere ( Fig. 1). These low concentrations are due to low water solubility of HCB (5µg/liters). Thus HCB does not directly discharge into water. HCB releases to water include direct point source discharges to water bodies as well as contamination via agricultural runoff from the use of pesticides containing HCB. According to toxic release inventory (TRI) data pesticide and chloralkali production facilities periodically discharge HCB to local water bodies. In 1997, 250 lbs of HCB were released into water by the alkalies and chlorine sector and 26 lbs by the agricultural chemicals sector, where only 4 lbs of HCB is released directly into the water.
MODE OF ACTION
Although HCB is persistent, it does degrade at a slower rate in all environmental compartments. This degradation depends on half-life of HCB in various compartments. If it is released to the soil, it has a half-life of 3-6 years. This means that half of the total amount will disappear after 3-6 years, half of the remaining amount will disappear in another 3-6 years, and this process will continue each 3-6 years thereafter. If it is released to surface waters such as lakes, rivers, and streams, the halflife is 2.7-5.7 years, and if it is released to groundwater, the half-life is 5.3-11.4 years. Its half-life in air ranges from 0.63 to 6.28 years.
Once HCB enters the environment, it breaks down very slowly, making it extremely persistent. One reason for its slow degradation is that it does not dissolve in water, mostly sticks strongly to soil particles as well as to sediment on the bottom of lakes and rivers. It can also build up in wheat, grasses, some vegetables, and other plants when it is present in soil (NRDC, 2001).The major route of human exposure is through food.
Hexachlorobenzene can enter our body when we eat food contaminated with it. After it enters the body, it rapidly spreads through the blood to many tissues in the body, especially to fat. This probably happens within a few hours. It will remain in the body, especially in fat, for years. During pregnancy, this substance can transfer to the fetus through the mother's blood, or after birth large amount of HCB can also be transferred through breast milk. Most of the HCB is eliminated from body through feces and urine (ATSDR, 1996).

TOXICITY
The first toxicity on HCB exposure was reported during 1954-1959 when people of Anatolia, Eastern Turkey developed porphyria turcica, a disorder of heme biosynthesis and porphyria cutanea tarda. 500 people were fatally poisoned and more than 4,000 people felt ill by eating bread made with HCB-treated flour that was intended for agriculture use. Most of the people were affected with porphyria cutanea tarda, which disturbs the metabolism of hemoglobin and results in skin lesions. Mortality was recorded up to 14%. Mothers, who had eaten tainted bread, transfer the HCB to their children by placental transfer and through breast milk. Children born to these women developed "pembe yara" or pink sore and died within 2 years (Peters, 1976).
The women who survived the Turkish HCB episode were identified as an appropriate group to evaluate for human reproductive outcomes (John Jarrell et al., 1998). A study on the toxic profile of HCB was conducted with 42 subjects affected with Porphyria Cutanea Tarda. Meanwhile, another 42 subjects were selected from the same region of Diyarbakir in southeastern Turkey who had not exposed to HCB (control group). In addition, 42 subjects were obtained from the capital, Ankara (control group). After 40 years of studying John Jarrell et al., 1998 noticed that the control subjects did not differ from the exposed group in the mean serum concentrations of HCB. From this he concluded that HCB had been effectively eliminated from the exposed population, or the HCB is transferred over a long distance and exposed to control groups. Follow-up studies conducted by Jensen et al., 1991 concludes that average HCB levels in breast milk were still more than seven times the average for unexposed women.
EXPOSURE AND HEALTH EFFECTS
People were exposed to these contaminants primarily through the foods contaminated with HCB as a result of the accumulation of this substance in the high-fat foods, such as, dairy products, eggs, animal fats, and some fish. HCB is highly found to be accumulated in fat rich tissues, so marine mammals such as blue whales and seals will be highly exposed to HCB emissions (Espelend et al., 1997). In addition, HCB is poor water soluble, thus it concentrated on sea water and exposed to marine organisms through food chain. By eating fishes that were exposed to HCB, humans were also subjected to exposure. The infants receive large dose of HCB from their mother either through breast feed or via lipid rich eggs. Nakashima et al (1997) showed that rats transfer HCB to their offspring’s through breast feed.
About 90% of HCB has been intake by adults in the general population through diet. Based on levels of HCB in air, water and food, the total intake of HCB by adults is estimated to be between 0.0004 and 0.003 ng/g body weight per day (ATSDR, 2002). In Germany, the women who ate a healthy diet (with low meat consumption and high fruit and vegetable intake) had much lower levels of HCB in their milk compared to women who ate a lot of meat (Schade and Heinzow, 1998). HCB exposure occurs by industrial emissions also. Quinsey et al (1995) showed that areas with less industrialization have significantly lower levels of HCB in the general population, whereas people living in industrial areas have high concentration of HCB exposure.
Exposure to HCB may cause eye, skin, and respiratory tract irritation. Long-term oral exposure has been reported to cause liver disease with associated skin lesions such as porphyria cutanea tarda in humans (U.S.EPA, 2000). Porphyria is the most consistently identified outcome following exposure of humans with HCB. It was discovered that HCB induces Porphyria after outbreak of porphyria cutanea tarda (PCT) in Turkey between 1955 and 1959 (John Jarrell et al., 1998). The symptoms reported for this disease are neuritis, photosensitivity, fragile and scarred skin and increased excretion of porphyrins (Peters et al, 1982). Studies in animals and humans have provided inconclusive evidence of carcinogenicity for hexachlorobenzene. Animal studies have reported cancer of the liver, thyroid, and kidney from oral exposure to hexachlorobenzene. The International Agency for Research on Cancer (IARC) has determined that hexachlorobenzene is possibly carcinogenic to humans. They placed HCB in Group 2B (IARC, 1987). The U.S.EPA has concluded that hexachlorobenzene is a probable human carcinogen. They placed HCB in Group B2 (ATSDR, 2002).
SOURCES OF HCB HCB
dominated its emissions during the 1950s and 1960s due to its direct use as fungicide. During this period thousands of tonnes of HCB were used each year. This period is referred to as peak period of HCB emission. Details of the global production of HCB are not known for this peak period. Today, however, HCB is no longer used in agriculture. Its use as a fungicide was banned in most countries in the 1970s and 1980s. This period is referred to as peak decline period. During this period the largest single primary source of HCB in the environment was removed, and therefore emissions of HCB fell sharply in the 1980s. The graphical data showing significant decreases in HCB emissions in Europe from 1970- 1995 was shown in Fig. 3.
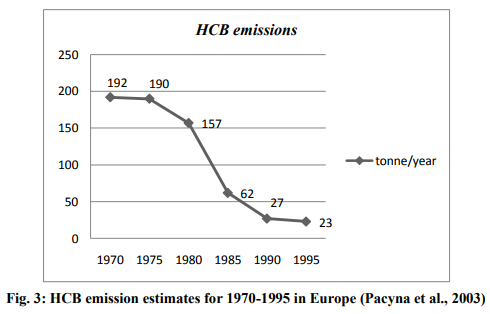
Currently, the principal sources of HCB in the environment are estimated to be the manufacturing of chlorinated solvents, the manufacture and application of HCB-contaminated pesticides, and inadequate incineration of chlorine-containing wastes (Bailey, 2001).
Industrial sources
The common route for industrial HCB production is the direct chlorination of benzene at 150-200°C over a ferric chloride catalyst or from the distillation of residues from the production of perchloroethylene (Brooks and Hunt, 1984). However this direct synthesis of HCB was banned in 1970, it is produced inadvertently by following industries.
i. The Metals Industry
The metallurgical processes such as high temperature steel production, electrolytic production of aluminium, the smelting and refining of copper are found to be typical sources of HCB. HCB is emitted to the atmosphere in form of flue gases generated by metallurgical industries (Bailey, 2001). Westberg et al. (1997) has reported that smelting of hexachloroethane (HCE) can produce HCB with an emission factor of 5.2mg of HCB/ton of aluminium. The smelting of magnesium is also believed to produce HCB in high rates. Surface coating of metal cans also will release significant amount of HCB. About 420 lbs i.e., 21% of total HCB emissions were found to be released from surface coating of metal cans (U.S.EPA, 2000).
ii. Pulp and Paper Mills
The manufacture of pulp and paper has resulted in HCB release into environment. Unlike poly chlorinated biphenyls (PCBs) pulp and paper mills do not favour production of HCB. But report from the Ontario Ministry of Energy and Environment, based on Municipal/Industrial Strategy for Abatement (MISA) states that pulp and paper mills take part in emitting HCB in limited amount (Jonathan Barber et al., 2005).
iii. Inorganic chemical industry
Silicon-based products such as antifoams, emulsions, hard coats, elastomers, adhesives, release coatings, and sealants, are reported as inorganic chemicals that releases HCB in large amount. These industries generate HCB by reducing silica (sand) to elemental silicon by reaction with methyl chloride at 300ºC in the presence of a copper catalyst. About 958 lbs i.e., 48% of total HCB emissions were found to be released from industrial inorganic chemicals (USEPA, 2000).
Combustion Sources
i. Cement Production
It is a controlled combustion process. Burning of hazardous waste as fuels for cement kilns has caused release of HCB into atmosphere. About 16% of the cement kilns burn hazardous waste as an auxiliary fuel. Data from a survey conducted by the Canadian Portland Cement Association states that during cement production about 0.17 mg of HCB is released per ton of cement (Bailey, 2001).
ii. Fires and open burning
It is an uncontrolled combustion process. Open burning of house hold waste plays a significant role in release of HCB. The amount of HCB emitted from residential open burning is estimated at 22-48 mg /ton of waste burned (Lemieux et al., 1999).
iii. Petroleum Refining The sources of HCB at petroleum refineries are not known accurately. A very limited literature is available for the emission of hexachlorobenzene from petroleum refineries. Catalytic reforming process is best example for Refining process. Here Regeneration of spent catalyst requires oxidative removal of contaminants at temperatures of 400- 455ºC and then reactivation of the catalyst through the use of chlorinated compounds (e.g., methylene chloride, 1,1,1-trichloroethane, and ethylene dichloride) at 400-500ºC. Due to heating of chlorine containing compounds at high temperature, it releases HCB and PCBs. About 32 lbs/year i.e., 1.6% of total HCB emissions were found to be released from this process (USEPA, 2000).
Incineration Sources
Incineration is an important source of HCB in the environment. HCB can be emitted from incineration as a result of incomplete thermal decomposition of HCB-contaminated industrial wastes, chlorinated organic compounds such as Kepone, mirex, chlorobenzenes, PCBs, PCP, PVC and mixtures of chlorinated solvents (Jonathan Barber et al., 2005). Municipal waste, medical waste and sewage sludge incinerators are major source of incineration process reported to emit hexachlorobenzene in large amount (Benazon, 1999). Total HCB releases from municipal incinerators in the US were estimated to be 977 kg/year in the early 1980s (Brooks and Hunt, 1984). HCB has been detected in emissions from the incineration process such as combustion of coal and hazardous wastes. HCB emissions from incineration facilities vary widely depending on the type of furnace, collection method and incineration temperature. There are many emission routes are possible from incineration. Among that most important emission route was concluded to be flue gas accounting about 62-97.9% followed by fly-ash and incinerator ash accounting about 1.9-38% and 0-2% respectively.
By-Product Sources
i. Pesticide Production:
Historically, HCB has been formed as a byproduct during the production of several chlorinated pesticides such as pentachlorophenol, dicloran, pentachloronitrobenzene (PCNB), chlorothalonil (TPN), trimethyl 2,3,5,6-terephthalate (TCTP), picloram and dacthal. These pesticides contribute bulk emission of HCB. Usually they found as an impurity in these pesticides (Ritter et al., 1995). But current applications of such pesticides are eliminated due to bans and restriction against HCB usage. When pesticides containing HCB are applied to crops, lawns, or gardens, HCB is released into the environment. The amount of contamination of HCB in various pesticides was estimated by Bailey (2001).
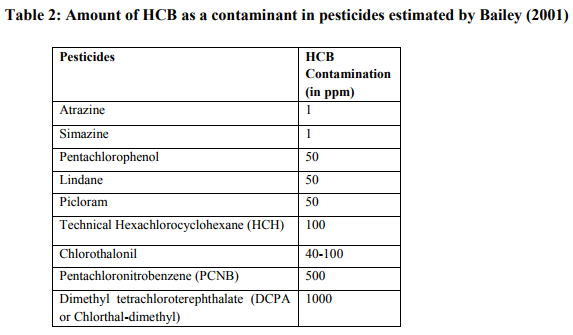
At present, all these pesticides except DCPA, picloram, and chlorothalonil have been banned in Europe. These pesticides contained HCB as an impurity in the final product. The impurity occurs when the appropriate procedures for synthesis and purifications were not followed. As a result, the level of HCB could be much higher (for example, PCNB was reported to contain 1.8-11% HCB (Tobin, 1986)). Almost every country including India has banned usage of these HCB contaminated pesticides, In Japan, HCB was never registered as an agricultural chemical, but during the period from 1952-1972, 70,000 tonnes of HCB was produced as a raw material for PCP, and this was probably the major source of HCB in that country.
ii. Chlorinated Solvent Production
Hexachlorobenzene is released as an unintentional by-product during manufacture of chlorinated solvents. Substantial quantities of HCBs are contained in the wastes generated by chlorinated solvent production. Perchloroethylene (PCE), Trichloroethylene (TCE), Carbon tetrachloride and pentachlorbenzene were major chlorinated solvents found to be emitting HCB in large quantities (Ritter et al., 1995). Even though, the HCB is removed by distillation of the solvents, the traces may still remain. The HCB can be separated by distillation process and settled in the distillation bottom fractions. The concentration of HCB in distillation bottoms in the 1980s was estimated to be 25% for perchloroethylene, 15% for carbon tetrachloride, and 5% for trichloroethylene (Jacoff et al, 1986). Manufacture of vinyl chloride monomer and volatile hydrocarbons is also known to produce HCB as a by-product. About 142 lbs/year i.e., 7% of total HCB emissions were found to be released from chlorinated solvent production (USEPA, 2000).
REMEDIATION AND REDUCTION OF HCB
Remediation involves removal of HCB from different compartments like soil sediments, air and water. Since, HCB was highly detected in soils and sediments (Fig.1). The majority of remediation technique adapted involves destruction of HCB from soils. HCB was settled down in soils for many years due to its long half life (more than 6 years) and affect the soil stability. There are several techniques to destroy the traces of HCB from soils and sediments. These treatment equally reduces the concentration of chemical congeners that are been produced by the HCB.
Base-Catalyzed Decomposition (BCD)
High grade impacted soils and wastes can be treated with this technology and contaminant load can be reduced by repeated treatment (Chen et al., 1997). BCD technology treats contaminated media by mixing it with an alkali (sodium bicarbonate) and heating them in a thermal desorption reactor to between 315 –500°C, which evaporates the halogenated compounds. The vapour stream is then condensed and sends to a BCD liquid tank reactor (LTR), where sodium hydroxide (catalyst) and carrier oils are added. The suspension in the reactor heated above 326°C for 3 - 6hrs, in which the contaminants are broken down. The technology can treat modest amounts of a wide range of soil sediments and mixed wastes, highly impacted with HCB to achieve very stringent clean-up targets if required by repeated application of the process to the same contaminated mass.
High-Temperature Dehalogenation (HTD)
This technology is used to treat high grade waste contaminated with pesticides and stockpiled material, by using the byproducts of calcium salt, elemental carbon and hydrogen, all of which are non-hazardous reaction products. It is effective only on HCB contaminated medium. In HTD process, the contaminated soils are mixed with calcium hydride or Ca2+ and placed in a reaction chamber containing a tungsten element, which is pressurized under pure argon gas and then electricity is pulsed through the tungsten coil to initiate the reaction. Once initiated, the temperature will reach to 3727°C. At such high temperature the POPs were break down into calcium chloride, carbon and H2. This technology has been proven at bench scale to destroy all POPs (mainly trialed on HCB).
GeoMelt techniques
GeoMelt can be used to treat very high concentration media and stockpiles, either in ex - situ or in -situ (Balmer et al., 2000; Goncalves et al., 2006). This technology uses intense heat to destroy HCB and permanently immobilize high grade wastes residues. The GeoMelt process uses a series of graphite electrodes and graphite frit within the soil matrix, placed in the ground. When matrix is heated between 1400 to 2000°C, the HCB break down typically to CO2, H2O and some HCl and volatilizes. The end product would be crystallized form of HCB and thus the media is completely free from HCB contamination. This technique is not suitable to treat soils below the water table or those having high water content.
High Temperature Incineration
The technology can treat small quantities of high grade to low grade soils, sediments and waters contaminated with HCB and PCBs. The process involves the heating of the contaminated media at 450-1100°C in a continuous indirect feed horizontal rotary kiln, under vacuum pressure below 50 kPa in the absence of air. The contaminants will vaporize and can be eliminated. This technique is used to remove HCB, PCBs, and dioxins. By this process contaminant samples of 0.1 ton/hr can be removed (Health and Safety Executive, 1993).
Gas Phase Chemical Reduction (GPCR)
The technology is used to clean-up impacted media with all forms of high grade POPs, at full scale to very low residual levels, in a sealed system. High grade POPs impacted soils, sediments and leachate/extracted waters can be treated by GPCR. The process uses 2 stages to achieve removal of HCB. In the first stage materials are heated to 600°C without oxygen in a batch process. This desorbs the POPs that volatilize and the treated soil is allowed to cool. In the second phase the gas vapours from the initial reaction pass to the GPCR reactor, where they react with H2 gas at 850–900°C. At this stage organic compound is converted to methane and water, chlorinated compounds are converted into HCL. Thus along with chlorinated compounds the HCB is eliminated. GPCR has been selected by UNIDO for pilot scale demonstration to treat 1,000 tons of HCB contaminated soil in Slovakia.
The major advantage in this process is that, it converts contaminants partly to CH4 which is then used as fuels.
Bioremediation process
Biological treatment includes usage microorganisms in the process that involves biodegradation. This biological treatment is a costly technique when compared to the incineration technique (Leagau, 1990). Biodegradation is a treatment process which uses microorganisms such as fungi and bacteria to degrade hazardous substances into nontoxic substances like water, CO2 (Ballerstedt et al., 1997; Mori and Kondo, 2002). After the HCB was degraded, the microbial population is reduced because they have completely utilized HCB as their food source. The extent of biodegradation is dependent on type of microorganism selected. Bioremediation can take place under aerobic and anaerobic conditions. In absence of oxygen, microorganism’s will breakdown HCB in the soils and sediments. In aerobic conditions, with sufficient oxygen, microorganisms will convert chlorinated compounds to carbon dioxide and water.
Solvent extraction process
Solvent extraction is a physico-chemical process adopted for separating organic contaminants from soil and sediment. By this process a considerable amount of chlorinated contaminant such as HCB that are present in soil and sediments are destroyed. In this process contaminated samples are fed into the extraction system along with solvent. Typically, more than 99% of the organics are extracted from the feed. In the extraction systems the solvent and organics are separated from the treated feed. After the separation the solvent and organic mixture passes to the solvent recovery system. In the solvent recovery system, the solvent is vaporized and recycled as fresh solvent. The organics are either reused or disposed off. Treated feed is discharged from the extraction system as slurry. The overall extraction efficiency of this process was found to be dependent upon the number of extraction cycles performed (Meckes et al., 1997).
CONCLUSION
With the knowledge of the various sources of the HCBs, action must be taken to reduce the exposure of such man-made chemicals. HCBs are considered as toxic by all routes of administration. Hematotoxicity and immunotoxicity have been consistently reported to be the most sensitive indicators of these chemical compounds. Therefore, more data are needed to establish the equivalency factors between their effects and their toxicity so as to allow normalization of measurements between different studies.
References:
1. Agency for Toxic Substances and Disease Registry (ATSDR). 1996. Toxicological Profile for Hexachlorobenzene (Update). Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA.
2. ATSDR. 2002. Toxicological profile for HCB. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (ATSDR), Atlanta, GA. http:/www.atsdr.cdc.gov.
3. Bailey RE. Global hexachlorobenzene emissions; Chemosphere, 2001; 43: 167-182.
4. Ballerstedt H, Kraus A, Lechner U. Reductive dechlorination of 1,2,3,4 - tetrachlorodibenzo-p-dioxin and its products by anaerobic mixed cultures from Saale river sediment. Environ. Sci. Technol. 1997; 31:1749–53.
5. Balmer ME, Goss KU, Schwarzenbach RP. Photolytic transformation of organic pollutants on soil surfaces —an experimental approach. Environ. Sci. Technol. 2000; 34:1240–5.
6. Benazon N. 1999. Hexachlorobenzene emissions/releases inventory for Ontario1988, 1998 and 2000, Draft Report for Environment Canada.
7. Berdowski JJM, Baas J, Bloos JPJ, Visschedijk AJH, Zandveldt PYJ. 1997. The European atmospheric emissions inventory of heavy metals and persistent organic pollutants; TNO Institute of Environmental Sciences, Energy Research and Process Innovation, The Netherlands.
8. Brooks G, Hunt G. 1984. Source assessment for hexachlorobenzene. Radian Corporation, Final Report. Prepared for US EPA, Research Triangle Park, NC.
9. Chen C M. The emission inventory of PCDD/PCDF in Taiwan. Chemosphere. 2004; 54:1413–20.
10. Elizabeth K, Douglas JG, Jim PP, Sherri EW. Gas phase chemical reduction of hexachlorobenzene and other chlorinated compounds: waste treatment experience and applications. 6th International HCH and Pesticides Forum, March 2001, Poznan, Poland
11. Espeland O, Kleivane L, Haugen S, Skaare JU. Organochlorines in mother and pup pairs in two Arctic seal species: harp seal (Phoca groenlandica) and hooded seal (Cystophora cristata). Mar. Environ. Res. 1997; 44: 315- 330.
12. Health and Safety Executive. 1993. Occupational Exposure Limits, EH40/93.
13. Hippelein M, McLachlan M S. Soil/air partitioning of semivolatile organic compounds. 1. Method development and influence of physical-chemical properties. Environ. Sci. Technol. 1998; 32: 310-316.
14. IARC. 1987. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1–42. Lyon, International Agency for Research on Cancer, pp. 219–229 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7).
15. Jacoff F S, Scarberry R, Rosa D. Source assessment of hexachlorobenzene from the organic chemical manufacturing industry. In: Morris C R, Cabral J R P. Hexachlorobenzene: Proceedings of an International Symposium. Lyon, IARC Sci. Publ. 1986; 77: 31-37.
16. Jensen AA, Slorach SA. Chemical Contaminants in Human Milk, 1991, Boca Raton Ann Arbor Boston: CRC Press, Inc.
17. Jonathan Barber, Andrew Sweetman, Kevin Jones. 2005. Hexachlorobenzene - Sources, environmental fate and risk characterisation - SCIENCE DOSSIER.
18. LEAGUE J. 1990. Liquid solids contact (LSC) or how to bud hazardous waste to death. In Innovative Hazardous Waste Treatment Technology. Vol. 3: Biological Processes (H.M. Freeman and P.R. Sferra, Eds), pp. 19 – 24.
19. Lemieux PM, Lee CW, Kilgroe JD, Ryan JV. 1999. Emissions of polychlorinated biphenyls as products of incomplete combustion from incinerators. PB-99-163065: 1-16.
20. Mackay D, Shiu WY, Ma KC. 1992. Illustrated Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals. Volume I. Monoaromatic hydrocarbons, chlorobenzenes, and PCBs. Lewis Publishers, Chelsea, Michigan.
21. Meckes MC, Tillman J, Drees L, Saylor E. Removal of PCBs from a contaminated soil using CF-systems(R) solvent extraction process. J. Air Waste Manage. Assoc. 1997; 47:1119–24.
22. Meijer SN, Shoeib M, Jantunen LMM, Jones KC, Harner T. Organochlorine pesticide in agricultural soils. 1. Field measurements using a novel in situsampling device. Environ. Sci. Technol. 2003; 37: 1292-1299.
23. Melnikova LV, Belyakov AA, Smirnova VG. and Kurenko LT. Sanitary-chemical methods for determination of noxious substances encountered in the production of sodium pentachlorophenolate. Gig. Tr. prof. Zabol. 1975; 7: 37–39.
24. Nakashima Y, Ohsawa S, Umegaki K, Ikegami S. Hexachlorobenzene accumulated by dams during pregnancy is transferred to suckling rats during early lactation. J. Nutr. 1997; 127: 648-654.
25. Natural Resources Defense Council (NRDC) 2001. Healthy Milk, Healthy Baby: Chemical Pollution and Mother's Milk. Retrieved September 13, 2008.
26. Pacyna JM, Breivik K, Munch J, Fudala J. European atmospheric emissions of selected persistent organic pollutants. Atmos. Environ. 2003; 37, 119-131.
27. Peters HA. Hexachlorobenzene poisoning in Turkey. Fed. Proc. 1976; 35, 2400-2403.
28. Peters HA, Gocmen A, Cripps DJ, Bryan GT, Dogramaci I. Epidemiology of hexachlorobenzene-induced porphyria in Turkey. Arch. Neurol. 1982; 39: 744-749.
29. Quinsey PM, Donohue DC, Ahokas JT. Persistence of organochlorines in breast milk of women in Victoria, Australia. Food Chem. Toxicol. 1995; 33: 49-56.
30. Ramamoorthy S, Ramamoorthy S. 1997. Chlorinated Organic Compounds in the Environment: Regulatory and Monitoring Assessment, Lewis Publishers.
31. Royal Haskoning 2003. Fact sheets on production, use and release of priority substances in The WFD, Hexachlorobenzene, Final Report, Brussels:European Commission, DG ENV, 12 p.
32. Schade G, Heinzow B. Organochlorine pesticides and polychlorinated biphenyls in human milk of mothers living in Northern Germany: current extent contamination, time trend from 1986 to 1997 and factors that influence levels of contamination. Sci. Total Environ. 1998; 215: 31-39.
33. Tissier C, Morvan C, Bocquené G, Grossel H, James A, Marchand M. 2005. Les substances prioritaires de la Directive cadre sur l’eau (DCE) : Fiches de synthèse, Report: IFREMER, 92 p.
34. Tobin P. Known and potential sources of hexachlorobenzene. In: Morris C R, Cabral J R P. (eds), Hexachlorobenzene: Proceedings of an International Symposium. Lyon, IARC Sci. Publ. 1986; 77: 3-11.
35. United States Environmental Protection Agency Persistent (USEPA) 2000. Bioaccumulative and Toxic Pollutants (PBT) HCB Workgroup, National Action Plan for Hexachlorobenzene for Public Review.
36. Westberg HB, Selden AI, Bellander T. Emissions of some organochlorine compounds in experimental aluminium degassing with hexachloroethane. Appl. Occup. Environ. Hyg. 1997; 12: 178-183.
37. WHO. 1997. Hexachlorobenzene (Environmental Health Criteria 195), Geneva, International Programme on Chemical Safety
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License