IJCRR - 6(1), January, 2014
Pages: 21-27
Print Article
Download XML Download PDF
A POSTMORTEM STUDY ON THE WEIGHT AND MORPHOLOGY OF ADRENAL GLANDS IN VICTIMS OF SUICIDE
Author: Arpita Sarkar, Manjari Chatterjee, Suddhodhan Batabyal
Category: Healthcare
Abstract:Objective: Stress plays an important role in affecting adrenal gland anatomy and structure which may have connection with suicidal behaviour in depressed patients. The increase in adrenal cortical function is paralleled by increased adrenal weight and zone specific cortical width. Method: Adrenal glands from persons aged 20-85 years who died unexpectedly either from road traffic accident or committed suicide and died immediately, were obtained from mortuary of Medical college and Hospital, Kolkata, within 48 hours of death and without any gross sign of putrefaction. After overnight fixation, the adrenals were carefully cleaned of adherent fat, blotted dry, weighed on electronic weighing machine having the accuracy of \? 0.1gram. After proper fixation, paraffin blocks were prepared, sectioned and stained with haematoxylin and eosin. Widths of cortical zones were measured by means of a calibrated eyepiece micrometer under light microscope. Result: The weight of right and left, both adrenal glands are significantly higher for suicide group than the control group. The width of zona fasciculata of right and left, both adrenal glands are significantly higher for suicide group than the accident group. The total cortical width of right and left, both adrenal glands are significantly higher for suicide group than the accident group. Conclusion: Increased weight of adrenal glands in suicide victims is mainly due cortical hypertrophy with increased width of zona fasciculata.
Keywords: Adrenal gland, suicide, weight, width of zona fasciculata, cortical width
Full Text:
INTRODUCTION
Suicide has taken lives around the world and across the centuries. It is one of the world’s largest public health problems and has multiple causes among which mood disorders contribute over 90% of suicide attempt and 60% of completed suicides (15). In India, 127151 and 134599 persons ended their life by a suicidal act in 2009 and 2010 respectively. This indicates an increase of 5.9% over the previous year's figure. West Bengal reported the highest number of suicidal deaths in 2008, and the second highest number of such deaths in 2010 accounting for 11.9%, while 11.4% of total such deaths in the country in 2009. (Source: National Crime Records Bureau, Accidental deaths and suicides in India, Ministry of Home Affairs, New Delhi, Government of India, 2010). Studies have shown that stress plays an important role in affecting adrenal gland anatomy and structure. In particular, adrenal gland size has been shown to be partially regulated by adrenocorticotropic hormone (ACTH) stimulation (3), which is known to regulate stress corticosterone levels during the hypothalamopituitary-adrenal (HPA) axis activation due to an acute physical or psychological stressor (26) . Elevated plasma cortisol levels and adrenal enlargement have been found individuals suffering from chronic stress, such as depressed patients (1, 17), and increases in adrenal weight have been found in individuals who have committed suicide (9, 11, 27). During acute stress, the elevated levels of corticosteroids induce a negative feedback loop to the pituitary gland, where they bind to glucocorticoid receptors to regulate the responsiveness of the stress system (6, 7). Chronic stress can lead to hypercortisolism or constant high levels of circulating glucocorticoids and dysregulation of the HPA axis, which can further affect the regulation of corticotropin-releasing hormone, catecholamines, and serotonin that are associated with the precipitation of depression (5, 8) . Previous studies showed that many patients with depression have increased basal plasma cortisol and enlarged adrenals (1, 4, 9, 10, 18, 19, 22, 27) . Depression is a chronic stress-related disorder and it is one of the most common causes of suicide. Many depressed patients have altered HPA axis function that is generally characterized by increased HPA axis activity (4, 19, 22) and enlarged adrenal glands (1, 9, 11, 24, 27) . Many studies showed that this increase in adrenal weight is associated with increased size of the adrenal cortex (27, 29) , and many depressed patients have exaggerated cortisol responses after ACTH administration (1, 13, 14) . Furthermore, increased glucocorticoid levels have been linked with the onset and severity of depression (6, 12) , suggesting that alterations in peripheral HPA axis structure and function may also be clinically relevant. Collectively, these works suggest that chronic stress-induced adrenal growth produces alterations in adrenal function that may have connection with suicidal behaviour in depressed patients (28) . The present work addresses the hypothesis that adrenal responses to ACTH are augmented after chronic stress and that this increased responsiveness is associated with increased weight and cortical hypertrophy and hyperplasia of adrenal glands.
MATERIAL AND METHOD
A cross-sectional descriptive type of study was designed and done in the Department of Anatomy, Kolkata Medical College, Kolkata, from April 2011 to October 2012, based on collection of human adrenal glands from 100 dead bodies that were under examination in the Department of Forensic Medicine, Kolkata Medical College, Kolkata from April 2011 to October 2012. This study was approved by the Ethical Review Committee of Kolkata Medical College, Kolkata. Both adrenal glands from persons aged 20-85 years who died unexpectedly either from road traffic accident or committed suicide and died immediately, were obtained from mortuary of Medical college and Hospital, Kolkata. All samples were collected within 48 hours of death and without any gross sign of putrefaction 24. We tried our best to exclude the cases of known adrenal abnormalities, chronic debilitating illness, or recent use of substances that might alter the hypothalamic-pituitary-adrenal axis function such as corticosteroids, antidepressants, and alcohol. After considering the inclusion and exclusion criteria, 100 adrenal glands were selected from victims of suicide who died immediately after hanging (Case) and 100 more adrenal glands were collected from victims of road traffic accident, died immediately on the spot of accident (Control). Among the 50 cases, 35 were males, 15females and among the 50 controls 36 were males and 14 females. The adrenal glands were removed and immediately placed in 10 % formal saline. After overnight fixation, the adrenal glands were carefully cleaned of adherent fat, blotted dry, weighed on electronic weighing machine having the accuracy of ± 0.1gram. Fixed weight does not differ significantly from fresh weight (2, 17, 23) . After a week fixation, paraffin blocks were prepared. Two to four blocks from each adrenal pair were selected for light microscopic examination and sectioned at 6micron. Sections were stained with haematoxylin and eosin (H and E). Widths of cortical zones were measured in each gland by means of a calibrated eyepiece micrometer. Ten measurements of the width of three cortical zones, the zona glomerulosa, the fasciculata, and the zona reticularis were made from each gland. The criteria by which the zones were defined were that the zona glomerulosa lies under the capsule and consists of patchy nests of cells with deeply staining nuclei, the zona fasciculata consists of parallel columns of cells with pale vacuolated cytoplasm surrounded by narrow sinusoids, and the zona reticularis consists of an irregular pattern of groups of deeply stained cells 16. Precautions observed in the choice of the parts of the sections from which the data were obtained were that they were always at right angles to the surface of the gland, and that infoldings of the gland were avoided because it was observed that the cortex appeared to be distorted and to be thicker in the folds than elsewhere 16. The slides having any adrenal pathology were also excluded from our study after confirmation from pathologists of the Department of Pathology, Kolkata Medical College, Kolkata. The obtained data was subjected to extensive analysis using Microsoft Office and S.P.S.S 16.0 version of software. Comparison of parameters between cases and controls was done by Student –t test.
RESULT
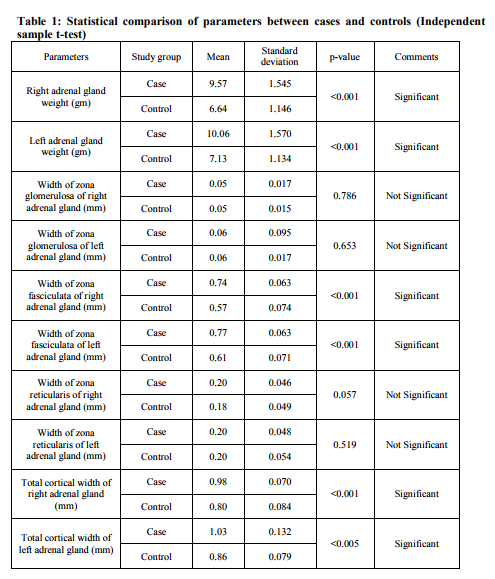
Among the 50 cases, 35 were males, 15 females and among the 50 controls 36 were males and 14 females. The right adrenal gland mean weight for suicide group was 9.57 grams and for control group was 6.64 grams, and left adrenal gland mean weight for suicide group was 10.06 grams and for control group was 7.13 grams. The mean± standard deviation (SD) weight of right and left, both adrenal glands were significantly higher for suicide group than the control group. The width mean of zona fasciculata of right adrenal gland for suicide group was 0.74 mm. and for control group was 0.57 mm. and that of left adrenal gland for suicide group was 0.77 mm. and control group was 0.61 mm. The mean±SD width of zona fasciculata of right and left, both adrenal glands were significantly higher for suicide group than the control group. The total cortical width (mean±SD) of right adrenal gland for suicide group was 0.98±0.070 mm. and for control group was 0.80±0.084 mm. and that of left adrenal gland for suicide group was 1.03±0.132 mm. and control group was 0.86±0.079 mm. The total cortical width (mean±SD) of right and left, both adrenal glands were significantly higher for suicide group than the control group. There was no significant difference in width of zona glomerulosa and zona reticularis between suicide group and the control group (p value >0.05, t test for unequal variance, two-tailed). The enlargement of cortex was mainly restricted to the zona fasciculata. [Table-1]
DISCUSSION
in our study we found that the adrenal weight, width of zona fasciculata, total cortical width were significantly higher for suicide group than the control group. Our findings support the findings of the earlier literatures regarding increased adrenal gland weight and altered adrenal morphology in suicide victims. Dorovini-Zis K, Zis AP (1987) had done a study on increased adrenal weight in victims of violent suicide among Canadian population, and adrenal gland weight was significantly higher in victims of violent suicide than who died suddenly from other cause. The mean±SD combined weight of both adrenal glands was significantly higher for the suicide group (9.77±1.74 g) than for the control group (7.74±0.82g) [p6 g/m2 may be a morphologic sign of a depressive disorder prior to death if no other disease with a known effect on the adrenals is present. A study done at University of Duesseldorf, Germany by Willenberg et al. (1998), on morphological changes in adrenals from victims of suicide. They found a significant enlargement of the adrenal cortex to 158.8% (SD = 29.8%, p < 0.01) that was restricted to the two inner zones only (zona reticularis, 161.6 +/- 35.3%; zona fasciculata, 186.4 +/- 34.4%). Szigethy E et al. (1994) had done a study on ‘Adrenal weight and morphology in victims of completed suicide’ and their results showed a positive correlation between adrenal weight and total cortical thickness in both left and right glands, providing direct evidence that increased adrenal weight in suicide victims is due to cortical hypertrophy. A study done at University of Duesseldorf, Germany by Willenberg et al. (1998), on morphological changes in adrenals from victims of suicide. They found a significant enlargement of the adrenal cortex to 158.8% (SD = 29.8%, p < 0.01) that was restricted to the two inner zones only (zona reticularis, 161.6 +/- 35.3%; zona fasciculata, 186.4 +/- 34.4%). A Computed Tomographic study on adrenal gland enlargement in major depression done by Nemeroff et al.(1994), showed that adrenal volumes in the depressed patients were significantly increased when compared with those of normal controls. Rubin et al. done a study on Adrenal gland volume in major depression: relationship to basal and stimulated pituitary-adrenal cortical axis function. Their study revealed that mean adrenal volume in the depressives was significantly larger, by about 38%, than the adrenal volume of their matched controls 21 . According to us it was very difficult to obtain an intact whole adrenal gland from post-mortem subjects. Many samples were rejected due to putrefactive changes as adrenal gland is one of the organs which undergo putrefaction very early. Weied our best to collect the samples within 48 hours of death and without any gross sign of putrefaction. We also tried to collect the past history of any depression or other mental diseases, any other chronic systemic diseases, history of prolonged intake of steroids or alcohol which might alter the hypothalamic-pituitary-adrenal axis function. But the information in many cases was not complete or reliable enough to warrant a statement regarding the presence and duration of specific psychiatric syndromes before the suicide. Considering that none of our subjects was suffering from a severe, protracted, or debilitating physical ailment at the time of death, we also assume that the observed increase in adrenal weight in our suicide group is largely accounted for by the pre-existing psychiatric disorders.
CONCLUSION
From above discussion we can conclude that depressed patients, who had committed suicide, have increased size of adrenal glands and this increased weight is due to hypertrophy of adrenal cortex which is evident by increased width of zona fasciculata.
ACKNOWLEDGEMENT
The authors of this article acknowledge the inspiration and help received from the scholars whose articles have been cited in the reference section. The authors pay their gratitude to authors/editors/publishers of all those /journals/books from where the reviews and literatures for the discussion have been collected.
References:
REFERENCES
1. Amsterdam, J.D. et al. (1987). Assessment of adrenal gland volume by computed tomography in depressed patients and healthy volunteers: A pilot study. Psychiatry Res., 21: 189-197
2. Beattie, M. K. and Heasman, M. A. (1958): The pituitary and adrenal glands of elderly mental hospital patients with and without hypertension. J. Path. Bact., 75, 83-94.
3. Bransome, Jr, E.D. (1968). Regulation of adrenal growth. Differences in the effects of ACTH in normal and dexamethasonesuppressed guinea pigs. Endocrinology, 83: 956-964.
4. Carroll BJ, Curtis GC, and Mendels J. (1976). Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry 33: 1051–1058.
5. Chrousos, G.P. (1998). Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann. N.Y. Acad. Sci., 851: 311-335.
6. De Kloet, E.R. et al. (1998). Brain corticosteroid receptor balance in health and disease. Endocrine Rev., 19: 269-301.
7. De Kloet, E.R., Joëls, M., and Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci., 6: 463-475.
8. De Rijk, R.H. et al. (2008). Corticosteroid receptor-gene variants: Modulators of the stress-response and implications for mental health. European Journal of Pharmacology, 585: 492-501.
9. Dorovini-Zis K, Zis AP. (1987) Increased adrenal weight in victims of violent suicide. Am J Psychiatry;144:1214-1215.
10. Dorovini-Zis K, Barocka A, Schubert E.(1998). Weight of adrenal glands may be increased in persons who commit suicide. Am J Forensic Med Pathol;19:72-76.
11. Dumser, Thomas; Barocka, Arndt; Schubert, Evelyn (March 1998) - Weight of Adrenal Glands May Be Increased in Persons Who Commit Suicide - American Journal of Forensic Medicine and Pathology: - Volume 19 - Issue 1 - pp 72-76
12. Gibbons JL. (1964) Cortisol secretion rate in depressive illness. Arch Gen Psychiatry 10: 572–575.
13. Jaeckle RS, et al. (1987). Enhanced adrenal sensitivity to exogenous cosyntropin (ACTH alpha 1–24) stimulation in major depression. Relationship to dexamethasone suppression test results. Arch Gen Psychiatry 44: 233– 240.
14. Kalin NH, et al. (1987). Function of the adrenal cortex in patients with major depression. Psychiatry Res 22: 117–125.
15. M. Pompili et al. "The hypothalamicpituitary-adrenal axis and serotonin abnormalities: a selective overview for the implications of suicide prevention."European archives of psychiatry and clinical neuroscience 260.8 (2010): 583-600.
16. Mackinnon, P. C. B., and I. L. Mackinnon. "Morphologic features of the human suprarenal cortex in men aged 20-86 years." Journal of Anatomy 94, no. Pt 2 (1960): 183.
17. Nelson A. Gelfman.(1964). Morphological change of adrenal cortex in disease; Yale journal of biology and medicine, Volume 37, August, 1964
18. Nemeroff CB, et al. (1992). Adrenal gland enlargement in major depression; a computed tomographic study. Arch Gen Psychiatry. 1992;49:384-387.
19. Rubin RT, et al. (1987). Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Arch Gen Psychiatry 44: 328–336.
20. Rubin RT, et al. (1995). Adrenal gland volume in major depression. Increase during the depressive episode and decrease with successful treatment. - Arch Gen Psychiatry. 1995 Mar;52(3):213-8.
21. Rubin RT, et al. (1996). Adrenal gland volume in major depression: relationship to basal and stimulated pituitary-adrenal cortical axis function. - Biol Psychiatry. 1996 Jul 15;40(2):89-97.
22. Sachar EJ, (1973). Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry 28: 19–24.
23. Sarason, E. L.(1943): Adrenal cortex in systemic disease. Arch. intern. Med., 1943, 71, 702-712.
24. Siddiqua, Dilruba, Shamim Ara, Abu Sadat Mohammad Nurunnabi, Rukshana Ahmed, and Ara Parven Hosne. "A postmortem study on the weight of the human adrenal glands." Bangladesh Journal of Medical Science 9, no. 4 (2010): 204-207.
25. Stein E,(1993). Adrenal gland weight and suicide. - Can J Psychiatry. 1993 Oct;38(8):563-6.
26. Solberg, L.C., et al. (2006). Genetic analysis of the stress-responsive adrenocortical axis. Physiol. Genomics, 27: 362-369.
27. Szigethy E, et al. (1994). Adrenal weight and morphology in victims of completed suicide. Biol Psychiatry.36:374-380.
28. Ulrich-Lai YM, et al. (2006). Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. American Journal of Physiology - Endocrinology and Metabolis,Published 1 November 2006Vol. 291no. E965-E973
29. Willenberg HS, et al.(1998). Morphological changes in adrenals from victims of suicide in relation to altered apoptosis. Endocr Res 24: 963–967.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License