IJCRR - 6(7), April, 2014
Pages: 74-78
Print Article
Download XML Download PDF
TRANSITIONAL CELL CARCINOMA OF THE OVARY: A RARE CASE REPORT
Author: Madhavan Manoharan, Sridevi Manian, Deepa Ganesh
Category: Healthcare
Abstract:Introduction: Transitional cell carcinoma of the ovary is a rare, recently recognized, subtype of ovarian surface epithelial carcinoma. Transitional cell carcinoma is sufficiently different from malignant Brenner tumour (MBT) in that it is reasonable to suppose that ovarian TCC arises directly from pluripotential surface epithelium of the ovary and from cells with urothelial potential, rather than from a benign or proliferative Brenner tumor precursor. Case presentation: We present a case of TCC of the ovary, managed by total abdominal hysterectomy and bilateral salpingo-oophorectomy with omentectomy. Conclusion: Primary TCC of the ovary is a rare subtype of epithelial ovarian tumours and their response to chemotherapy is better than that for other types of ovarian cancers.
Keywords: Transitional cell carcinoma, malignant Brenner tumour, pluripotential surface epithelium, total abdominal hysterectomy, bilateral salpingo-oophorectomy with omentectomy.
Full Text:
NTRODUCTION
Transitional cell carcinoma (TCC) of the ovary is a rare subtype of ovarian surface epithelial tumours. Incidence of occurrence is less than 1% of Primary ovarian carcinomas1 . Admixture of other types of surface epithelial tumours, including serous, endometrioid and undifferentiated carcinoma are common, but transitional cell carcinoma pattern must predominant (>50%)of the tumour to make the diagnosis2,3 .
CASE PRESENTATION
A 40-year-old woman presented with history of lower abdominal pain and dysuria on and off. On per abdominal examination, there was a pelvic mass on the right side. Ultra sonogram of the abdomen showed a pelvic mass measuring 31 × 35 mm with homogeneous echogenicity. Abdominal computed tomography also showed a right adnexal mass measuring 4×3 x2 cm separate from ovary. A possibility of fibroid in the right broad ligament was considered. There was no lymphadenopathy present. The liver and kidneys were unremarkable. Routine laboratory results were all within normal ranges. Initial investigation of tumour marker showed increased serum CA-125 (70.3 U/mL; normal, 0-35 U/mL). She underwent surgery under the impression of malignant ovarian tumour. A solid mass, arising from the right ovary was found. The other ovary, uterus, both tubes and broad ligaments were within normal limits. Therefore, a staging laparotomy including total abdominal hysterectomy, bilateral salpingooophorectomy and omentectomy was performed. No lymph nodes were present. Intraoperative, a small amount (about 100 ml) of fluid was found in the pelvic cavity and the same was sent for cytological examination.
PATHOLOGICAL EXAMINATION
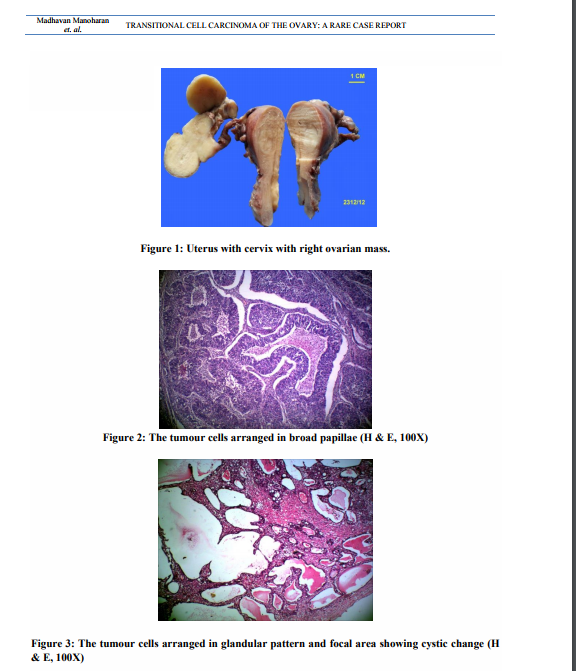
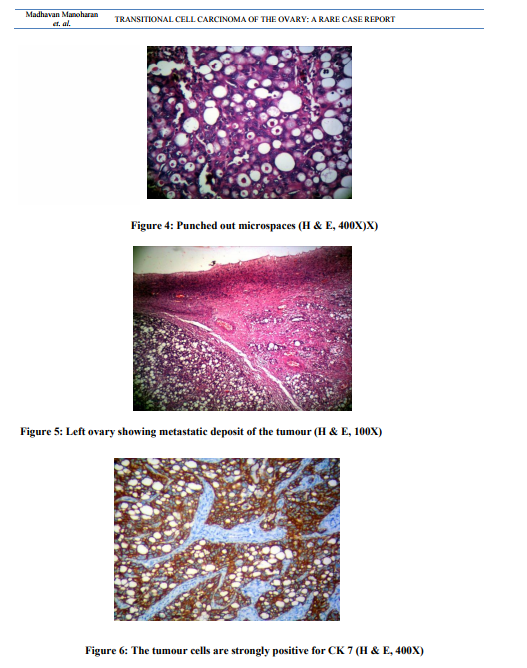
Uterus with cervix measured 7.5x4.5x3 cm. Cervix appeared hypertrophied. Cut section showed a thinned out endometrium and an intramural fibroid measuring 1.5cm in diameter. Each fallopian tube measured 5cm in length. The right ovarian mass measuring 6.5x3x2 cm was sent detached. The external surface was nodular. Cut surface was solid and yellowish (Figure 1). The left ovary measured 3.5x1.5x1cm. Cut section appeared within normal limits. Microscopic examination of the right ovarian mass showed a malignant tumour. The tumour was composed of oval to round cells with moderate amount of cytoplasm, vesicular to hyper chromatic nuclei and prominent nucleoli. The tumour cells were arranged in nesting pattern, broad papillae, glandular pattern and focal area shows cystic change lined by tumour cell (Figure. 2, Figure. 3). The tumour cells were separated by fibrous stroma and many punched out micro spaces were present (figure. 4). Mitosis was focally high, 5/10 HPF. There was no benign, metaplastic or proliferating Brenner component found in the tumour. The left ovary showed metastatic deposit of the tumour (Figure. 5). The tumour cells are strongly positive for CK 7 (Fig. 6). Sections of omentum showed lobules of adipose tissue with a focus of dystrophic calcification. No evidence of metastasis was present. The cytological examination of the fluid showed no malignant cells. The final diagnosis was transitional cell carcinoma of right ovary with metastasis to left ovary.
DISCUSSION
TCC of the ovary is a recently recognised subtype of ovarian surface epithelial carcinoma4 . It was first defined by Austin and Norris5 and added to the histologic classification of ovarian tumours by the World Health Organisation/International Society of Gynaecologic Pathologists 5 . Transitional cell tumour of ovary is a category of ovarian tumours that have epithelial cells resembling those of transitional cell neoplasms of urinary tract. They consist of Brenner tumour (benign, proliferating and malignant) and transitional cell carcinoma. World Health Organization classification defines ovarian transitional cell tumours as those “containing epithelial cells resembling urothelial transitional cells” and TCC as an invasive tumour that lacks a component of benign Brenner tumour and is characterized by “the presence of papillae lined by malignant cells of transitional cell type or nests of such cells in a fibrous or fibromatous stroma.” Ovarian TCC was thought to arise directly from the pluripotential surface epithelium of the ovary and from cells with urothelial potential, rather than from a benign or proliferative Brenner tumour precursor. Incidence of TCC is less than 1% of ovarian tumours. 10 to 15% of advanced stage ovarian carcinoma contains a TC component2 . They predominantly occur in post-menopausal age group, and the mean age is 56 years1 . The mean size of the tumour is 10 cm. In most of the patients, they are solid and cystic. But it can also present as purely solid or purely cystic with soft, fleshy or papillary areas often with haemorrhage and necrosis6 . They are unilateral or bilateral.Bilateralism is seen in 15 to 20% of cases (1). In our patient, the tumour was grossly seen only in the right ovary. The contralateral ovary appeared normal in size. However, it showed tumour deposit microscopically and hence considered as metastasis. The most important microscopic feature is the „punched out? micro spaces (87%). Other common features are large cystic spaces (73%) and large blunt papillae (63%). The less commonly occurring microscopic features are slit-like fenestrations (49%), bizarre giant cells (35%), small filiform papillae (18%), gland-like tubules (17%), and squamous differentiation (13%), and psammoma bodies (4%). The lining cells are crowded, highly stratified, polygonal to spindled cells with scant eosinophilic or clear cytoplasm and mild to severe nuclear atypia. Necrosis is common. In addition to not having a benign Brenner tumour component, TCC lacks the prominent stromal calcification which is mainly seen in malignant Brenner tumour7 . The micro spaces present in TCC may be mistaken for an endometrioid or a serous carcinoma. The former will show squamous differentiation associated with endometriosis whereas the latter will show complex papillae lacking transitional like cells and often contain Psammoma bodies1 . The ovarian TCC can be differentiated from an urothelial tumour metastatic to the ovary with the help of clinical findings and immunohistochemistry. Though both tumours can express cytokeratin 7, the ovarian TCCs are negative for cytokeratin 20 which is usually detected in bladder TCCs. Unlike bladder TCCs, ovarian TCCs are positive for Wilms tumour antigen (WT1)1, 2, 6 . It is well known that primary ovarian TCC has a better prognosis than all other types of ovarian carcinomas following standardized chemotherapy8 . Hence it becomes essential that the ovarian TCC has to be diagnosed correctly.
CONCLUSION
This case report will bring awareness of the rare and recently introduced entity, so that a tumour with urothelial features will be diagnosed properly and appropriately managed.
References:
REFERENCES
1. Christopher P. Crum. Transitional cell tumours. In DiagnosticGynaecologic and Obstetric Pathology, 2nd Edition. Marisa R. Nucci, and Kenneth R. Lee, 2011, 882-887.
2. AncelBlaustein. Transitional cell tumours. In Blaustein'sPathology of the Female Genital Tract, 5th Edition .Robert J. Kurman, 2002, 881-884
3. Christopher D. M. Fletcher. Transitional cell tumours. In Diagnostic Histopathology of Tumors , 4th Edition, William R. Schmitt , Kathryn DeFrancesco, 2013, 685-686, Volume 1.
4. Satoshi Ichigo, Hiroshi Takagi, KazutoshiMatsunami, Takayuki Murase, Tsuneko Ikeda, Atsushi Imai. Transitional cell carcinoma of the ovary (Review). Oncology Letters. 2011:3-6.doi: 10.3892/o1.2011.453
5. Austin RM, Norris HJ. Malignant Brenner tumor and transitional cell carcinoma of the ovary: a comparison. Int J GynecolPathol. 1987;6:29–39. doi: 10.1097/00004347- 198703000-00004
6. Scully RE. Histological Typing of Ovarian Tumours, 2nd ed. Berlin: Springer-Verlag, 1999.
7. Juan Rosai.Transitional cell tumours. In Rosai and Ackerman's Surgical Pathology, 10th Edition. Michael Houston, Joanne Scott, 2011, 574- 1576, Volume 2.
8. Eichhorn JH, Young RH. Transitional cell carcinoma of the ovary: a morphologic study of 100 cases with emphasis on differential diagnosis. Am J SurgPathol. 2004;28:453–63. doi: 10.1097/00000478-200404000-00004
9. Kommoss F, Kommoss S, Schmidt D, Trunk MJ, Pfisterer J, du Bois A. ArbeitsgemeinschaftGynaekologischeOnkolog ieStudiengruppeOvarialkarzinom. Survival benefit for patients with advanced-stage transitional cell carcinomas vs. other subtypes of ovarian carcinoma after chemotherapy with platinum and paclitaxel. GynecolOncol. 2005;97:195–9. doi: 10.1016/j.ygyno.2004.12.047
10. Satoshi Ichigo, Hiroshi Takagi, KazutoshiMatsunami, Takayuki Murase, Tsuneko Ikeda, Atsushi Imai. Transitional cell carcinoma of the ovary (Review). Oncology Letters. 2011:3-6.doi: 10.3892/o1.2011.453
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License