IJCRR - 6(7), April, 2014
Pages: 11-16
Print Article
Download XML Download PDF
ANTIOXIDANT AND ANTIFUNGAL ACTIVITIES OF METHANOLIC EXTRACT OF CHAETOMORPHA LINUM FROM INDIAN SOUTHEAST COAST
Author: Durga Devi V., Minhajdeen A., Pavithra V., Brindha S., Sree Jaya S., Saranya R. S., Varun S., Senthilkumar P., Sudha S.
Category: General Sciences
Abstract:Aim: Sea weeds are vital part of complementary and unconventional medicine suitable for the ability to generate secondary metabolites that are used to re-establish health and to treat many diseases. The present study aims at determinining the antioxidant and antifungal activities of the green seaweed Chaetomorpha linum. Methodology: Chaetomorpha linum, collected from the Gulf of Mannar, regions of Mandapam coastal area, Southeast coast of India were reported foremost for the antioxidant and antifungal activities based on the free radical-scavenging activity of the 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH), ferrous reducing antioxidant property (FRAP), and total phenolic content in the methanolic extract. The antifungal properties of the methanolic extract of C. linum were tested against pathogenic fungal strains like Fusarium dimerum and Trichoderma ressei. Results: The DPPH scavenging activity was equivalent to an IC50 value of 8.8 \?g/mL ascorbic acid. The total phenolic content was 668.2 mg/g gallic acid equivalent, and the IC 50 value by FRAP assay was 8.6 \?g/mL. On further examination, when compared with standard antibiotics, C. linum extract be a sign of considerable activity against Fusarium dimerum and Trichoderma ressei. Conclusion: Our findings represents C. linum is a natural antioxidant and a potential natural source of antifungal agents against selected fungal pathogens.
Keywords: Sea weeds, antifungal activity, DPPH activity, antioxidants
Full Text:
INTRODUCTION
Natural antioxidants are paid much consideration in the field of biomedicine with their association on health benefits (1). Many reports suggest that seaweed as a potential source of natural antioxidants as of their biological activities (2, 3). Among prospective features of marine algae and its components, quite a lot of extracts have been screened with regard to antioxidant and radical scavenging activity using stable free radicals (4-7). Seaweeds are used in the treatment of various infectious diseases traditionally. For many years, a mixture of synthetic chemicals used as antifungal agents inhibits the growth of plant pathogenic fungi. However, there are serious problems on the effective use of these chemicals (8, 9). Many studies proved that there are a large number marine species showing antifungal activities which in future throws more light on the use of marine algae by the pharmaceutical technologies for the extraction of useful drugs (10, 11). Furthermore, several groups of green, red, and brown seaweed have investigated for various properties (12, 13). Majority of seaweeds from the Gulf of Mannar has not been examined for their bioactive substances. Despite of the abundance and diversity of algae in coastal waters, Chaetomorpha linum (Class: Ulvophyaceae; Order: Cladoporales; Family: Cladoporaceae) is widespread in the Mandapam coastal region of the Gulf of Mannar on southeast coast of India which is mainly used as food, animal feed, and agriculture and until now, no screening of antioxidant activities has been performed. In this investigation, we evaluated the antioxidant and antifungal activity of a methanolic extract of C. linum, obtained from the Gulf of Mannar, a southeastern coastal region of India.
MATERIALS AND METHODS
Sample collection and preparationKutzing green seaweed C. linum collected from the Mandapam coastal region (78 ?8’E, 9?17’N), in the Gulf of Mannar, Tamilnadu, South India, on low tide in December 2012 was brought immediately to the laboratory in polythene bags which was washed several times with seawater to remove sand, mud, and attached fauna. The algae were cleaned using a brush to remove epiphytes with distilled water. After cleaning, it was dried in the shade at room temperature for 1 week. The dried algal materials were homogenized to a fine powder and subjected to extraction. Preparation of extracts Five hundred grams of powdered C. linum seaweed sample was taken and extracted successively with methanol (90%) using a soxhlet apparatus. The crude extracts were later concentrated under reduced pressure to obtain their corresponding residues. The methanolic extracts were further subjected to antioxidant and antifungal assays in triplicate. Radical scavenging assay The radical-scavenging activity of methanolic C. linum extracts against DPPH radicals was determined by the method of Blois et al (14). DPPH (0.1 mM in methanol) was prepared, and 1.0 mL of this solution was added to 3.0 mL of extract in methanol at various concentrations (1-16 μg/ mL). Thirty minutes later, the absorbance was measured at 517 nm. A blank was prepared ithout extract. Ascorbic acid at various concentrations (1 to 16 μg/mL) was used as the standard. A lower absorbance of the reaction mixture indicates greater free radical-scavenging activity. The ability to scavenge DPPH radical was calculated using the following equation: DPPH Scavenged (%) = A control– A test / A control X100 where A control is the absorbance of the control reaction and A test is the absorbance in the presence of the extracts. The antioxidant activity of the C. linum extract was expressed as IC50 and compared with the standard. The IC50 value was defined as the concentration (in μg/mL) of extract that inhibited the formation of DPPH radicals by reducing power assay. The reducing power of methanolic extracts of C. linum was determined (15). Various concentrations of the extracts (1-16 μg/mL) in 1.0 mL of deionized water were mixed with phosphate buffer (2.5 mL) and potassium ferricyanide (2.5 mL). The mixture was incubated at 50°C for 20 min, and aliquots of trichloroacetic acid (2.5 mL) were added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 mL) was mixed with distilled water (2.5 mL) and freshly prepared ferric chloride solution (0.5 mL). The absorbance was measured at 700 nm. A blank was prepared without extract. Ascorbic acid at various concentrations (1 to16 μg/mL) was used as the standard. Increased absorbance of the mixture indicates an increase in reducing power. % Increase in Reducing Power = A test / A blank - 1 x 100 where A test is the absorbance of the test solution and A blank is the absorbance of the blank. The antioxidant activity of the seaweed extract was expressed as IC50 and compared with the standard.
Determination of total phenolic content
Total phenolic content of the C. linum extracts was determined using the Folin-ciocalteu reagent (16). One milliliter of extract in Gallic acid (20, 40, 60, 80, and 100 mg/L) was added to a 25 mL volumetric flask, containing distilled deionized water. The blank reagent was set with distilled deionized water. One milliliter of Folin-ciocalteu phenol reagent was added to the mixture and mixed by shaking. After 5 min, 10 mL of 7 % Na2CO3 solution was added to the mixture. The solution was diluted to 25 mL with deionized distilled water and mixed. After incubation for 90 min at room temperature, the absorbance against the prepared blank reagent was measured at 750 nm on a spectrophotometer. Total phenolic content of the seaweed was expressed as mg Gallic acid equivalents (GAEs) or 100 g fresh weight. All samples were analyzed in triplicate.
Antifungal activity
The following strains of fungi were used: Fusarium dimerum (MTCC 6583), and Trichoderma ressei (MTCC-3929) was obtained from the Institute of Microbial Technology, Chandigarh, India. Cultures were maintained on potato dextrose agar (Hi Media, India) slants at 4°C for further use. The extracts were tested for their efficiency against the fungal pathogens by using an agar dilution technique (18). Different concentrations of the extracts; 20%, 10%, and 5% were obtained by amending PDA. The amended medium was dispensed into sterile petri plates and allowed to solidify with streptomycin (100 µg/ml). Each plate was inoculated with F. dimerum and T. ressei. A 4-mm diameter mycelia disc of each of the test organisms was inoculated on each amended agar plate. Inoculated plates were incubated at 25 ± 2 oC and growth measured along the perpendicular lines. Daily radial growth of each test organism in any of the test extracts was recorded for 7 days. Each treatment was replicated thrice with appropriate untreated controls. In here three replications were prepared for each treatment. Then all the culture plates were incubated at 25 ± 2 oC in dark condition. The mycelia growth of fungus was measured after 96 hours. Percentageinhibition was calculated against the mycelia growth over control (19).
RESULTS
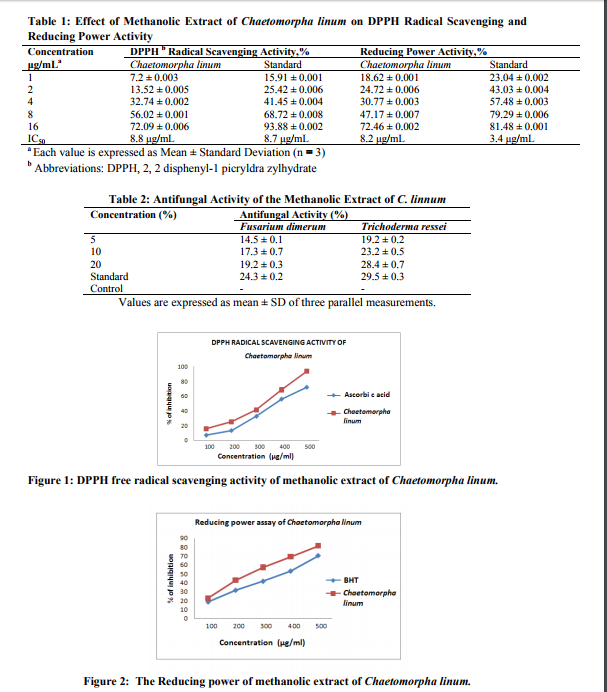
DPPH, a stable free radical, will decolorize in the presence of antioxidants on DPPH antioxidant assay. The comparison of the antioxidant activity of the extracts (at 1, 2, 4, 8, and 16 μg/mL) and reference standard is shown in Table 1. The methanolic extract of C. linum exhibited a significant dose-dependent inhibition of DPPH activity, with an IC 50 value 8.8 µg/mL. The IC 50 value of the extract was comparable with that of the reference standard, ascorbic acid (IC 50 = 8.7 µg/mL), indicating the antioxidant activity of C. linum (Figure 1). The IC 50 value in the reducing power assay was 8.2 µg/mL and 3.4 µg/mL, respectively, for the methanolic extract of C. linum and ascorbic acid (Figure 2). By Folin-Ciocalteu method, the highest total phenolic content of C. linum was 668.2 mg/GAE/100 g/extract. The methanolic extract of C. linum was tested for its antifungal activity. The results of the antifungal studies with regard to percentage of radial growth inhibition in PDA plat are shown in Table 2. All the extracts exhibited different degrees of antifungal activity against F. dimerum and T. ressei. The growth of T. ressei was highly inhibited by all the tested concentrations (5-20%) of methanol extracts of sea weeds compared with control, the corresponding inhibition ranging from 81% - 72%. The extract showed comparatively very low activity against F. dimerum ranging from 86% - 76%.
DISCUSSION
The detection of antioxidants from sea weeds is a fast-growing and many antioxidants have been investigated by several methods. The rapid, reliable, and economical method to evaluate the antioxidative potential of various natural compounds is through DPPH assay (18). Methanolic extracts of C. linum show signs of potent antioxidant activity in a dose-dependent manner, by DPPH radical scavenging assay. In the present study, the methanolic extract of C. linum contains a high amount of phenolic compounds which exhibited the greatest antioxidant activity. Reducing power is associated with antioxidant activity and the reduction of ferrous ion to ferric ion was calculated in the methanolic extract of C. linum. All concentrations of methanolic extract showed significant activity when compared with the standard, ascorbic acid. There was a concentration-dependent increase in the reducing power of methanolic extract of C. linum. Many earlier studies have proved for the antifungal effects of marine sea weeds (19, 20). The methanolic extract of C. linum showed strong antifungal activity against selected human pathogens. The largest zone of inhibition was observed against Fusarium dimerum and Trichoderma ressei. These results are notable, because they were obtained with methanolic extracts, which are not pure products but with superior effect. Considering the total phenolic content, reducing power, and DPPH radical scavenging activity, our findings reveal it as a prospective source of natural antioxidants, indicating that C. linum is capable of treating diseases that are related to free radical reactions. Our results prompt further studies to isolate and identify the active compounds that evaluate a possible synergism between components with regard to their antioxidant and antifungal activity. This work provides insight into the molecular basis of the therapeutic properties of C. linum in pharmaceutical industry. The antifungal study revealed that the methanolic extract of C. linum contains certain constituents with important antifungal properties. The overall results of the study revealed that the crude extract of marine sea weed can act as a potential for the studies on the isolation and characterization of the plant extract necessary to realize new biological antioxidants and antibiotics.
CONCLUSION
Results from this study exposed the antifungal property of the crude extract of seaweeds that contain certain constituents be a better alternative to the hazardous pathogens. In addition, it forms a basis for selection of this as in additional pharmacological investigation. C. linum is undergoing research with the aim of isolating biologically active molecules along with novel antifungal agents.
ACKNOWLEDGMENTS
The authors are grateful to the authorities of Karpagam University, Coimbatore, Tamil Nadu, India for providing facilities and for their encouragement. Authors also thank Dr. M. Ganesan, Scientist, CSMCRI- Marine Algal Research station, Mandapam camp, Tamilnadu, India for the species identification. Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
REFERENCES
1. Kalim MD, Bhattacharyya D, Banerjee A, Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement Altern Med. 2010; 10: 77.
2. Airanthi MK, Hosokawa M, Miyashita K. Comparative antioxidant activity of edible Japanese brown seaweeds. J Food Sci. 2011; 76: 104-11.
3. Devi GK, Manivannan K, Thirumaran G, Rajathi FA, Anantharaman P. In vitro antioxidant activities of selected seaweeds from Southeast coast of India. Asian Pac J Trop Med. 2011; 4: 205-11.
4. Cho SH, Kang SE, Cho JY, Kim AR, Park SM, Hong YK, et al. The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J Med Food. 2007; 10: 479-85.
5. Devi KP, Suganthy N, Kesika P, Pandian SK. Bioprotective properties of seaweeds: in vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement Altern Med. 2008; 8: 38.
6. Shanab SM, Shalaby EA, El-Fayoumy EA. Enteromorpha compressa exhibits potent antioxidant activity. J Biomed Biotechnol. 2011; 2011: 1-11.
7. Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol. 2006; 44: 1144-50.
8. Brent KJ, Hollomon DW. Fungicide Resistance: the Assessment of Risk. FRAC, Global Crop Protection Federation, Brussels. 1998; 48.
9. Schillberg S, Zimmermann S, Zhang MY, Fischer R. Antibody-Based Resistance to Plant Pathogens. Trans Res 2001; 10: 1-12.
10. Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003 Jul; 66: 1022-37.
11. Ömer E, Beyhan T. Antibacterial and Antifungal effects of some marine algae. Kafkas J Fac Vet Med. 2011; 17: 121-124.
12. Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol. 2008; 99: 2717-23.
13. Gonzalez del Val A, Platas G, Basilio A, Cabello A, Gorrochategui J, Suay I, et al. Screening of antimicrobial activities in red, green and brown macroalgae from Gran Canaria (Canary Islands, Spain). Int Microbiol. 2001; 4: 35-40.
14. Blois MS. Antioxidant Determinations by the Use of a Stable Free Radical. Nature. 1958; 181: 1199-200.
15. Tachakittirungrod S, Okonogi S, Chowwanapoonpohn S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007; 103: 381-8.
16. Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Lester P, editors. Methods Enzymol: Academic Press; 1999. p. 152-78.
17. Tortora GJ, Funke BR, Case CL. Microbiology: An Introduction, including Microbiology Place (TM) Website, Student Tutorial CDROM, and Bacteria ID CD-ROM 7th ed. edition. Benjamin Cummings Publishing SF, USA; 2001.
18. Senthilkumar P, Sudha S. Antioxidant and Antibacterial Properties of Methanolic Extract of Green Seaweed Chaetomorpha linum From Gulf of Mannar: SoutheastCoast of India. Jundishapur J Microbiol 2012; 5: 411-415.
19. Vivek M, Senthil Kumar P, Steffi S, Sudha S. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotech 2011; 3: 143-148.
20. Senthil Kumar P, Sudha S. Biosynthesis of silver nanoparticles From Dictyota bartayresiana extract and their antifungal activity. Nano Biomed Eng. 2013; 5: 72-75.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License