IJCRR - 6(8), April, 2014
Pages: 63-70
Print Article
Download XML Download PDF
PURE TONE AUDIOMETRIC EVALUATION IN NON-INSULIN DEPENDENT DIABETIC PATIENTS
Author: S. Vijayasundaram, P. Karthikeyan, V. Nirmal Coumare
Category: Healthcare
Abstract:The prevalence of hearing defects in the Indian population is estimated to be about 6%. The risk factors are age, noise, cardiovascular system disorders, Diabetes mellitus and social factors. Aims: The aims of the study were to find out the prevalence and type of hearing loss among diabetic subjects, to establish if there was a relationship between age, duration, severity and complications of Diabetes to the changes in hearing threshold and to establish if there was a relationship between height, weight, family history, diet, blood pressure, blood group and blood cholesterol. Materials and methods: This study included 100 diabetic patients (NIDDM) and 200normal subjects (controls) in the age group of 30 \? 59 years, and the controls were matched for age and sex. A detailed clinical examination was performed using a diabetic proforma and screened for complications. The diagnosis was established with the help of tuning fork tests and audiometric analysis. Diabetic patients were categorized into groups and subgroups and were analyzed for statistical significance. Results: It was found that diabetics have an increased mean threshold of hearing at higher frequencies than non-diabetics. The type of hearing loss is typically bilateral and symmetrical, involving higher frequencies. The complications of the disease, sex, weight, height, family history, diet, blood pressure, blood group and blood cholesterol had no significant correlation with the type of hearing loss and with mean average hearing threshold. Conclusion: A relationship exists between the hearing impairment in diabetic patients and other aspects of the disease, which include age, duration and control of Diabetes mellitus.
Keywords: Pure tone audiometry, Non-insulin dependent Diabetes mellitus, Hearing loss
Full Text:
INTRODUCTION
Diabetes mellitus is a chronic metabolic disorder with an incidence of 1-2% which is classified as non-insulin-dependent diabetes mellitus or insulindependent diabetes mellitus, which corresponds to the previous labels of adult onset diabetes mellitus (Type II) and juvenile onset diabetes mellitus (Type I). They are suspected to be two separate entities from the pathogenesis, with the non-insulin type probably the result of breakdown of interaction of regulatory mechanisms, while insulin-dependent diabetes mellitus may be related to partial destruction of B cells in the pancreas and may be modified by polygenic (suspected to be a specific HLA antigen) and environmental (thought to be a viral infection) factors. An autoimmune component is thought to be responsible for the destruction of the B cells. Pathologically it constitutes the triad of neuropathy, retinopathy and nephropathy.1 Complications from insulin-dependent diabetes are more commonly microvascular, resulting in small vessel disease of the kidneys, retina and the skin as well as neuropathy, and less commonly, macrovascular. Non-insulin-dependent diabetes is more common in obese and older patients with a strong genetic predisposition. Fewer microvascular complications are seen; there is a higher risk of large vessel atherosclerosis, coronary disease and peripheral vascular disease.2 The literature exhibits many contradictions concerning the correlation between hearing impairment and diabetic manifestations. It is evident that good control and regular surveillance for complications enables the patients to lead a normal life. The typical hearing loss described is a progressive, bilateral. Sensorineural deafness of gradual onset affects predominantly the higher frequencies and older patients. There is a decrease in auditory acuity which is similar to that of presbyacusis, but those affected show a hearing loss greater that that expected at that age.3,4 Worldwide the prevalence of hearing impairment is estimated at about 440 million people. Age is the primary risk factor in the population. Other risk factors include noise, cardiovascular disease, Diabetes mellitus and social factors. Non-insulin dependent Diabetes mellitus accounts for almost three quarters of all cases of diabetes. Angiopathy and peripheral neuropathy are well recognized complications. It is reasonable to expect these lesions to affect the inner ear leading to hearing impairment.8,9,10 The oculomotor, trochlear and facial nerve palsies seen in diabetic subjects further suggest that besides autonomic and peripheral neuropathy there is definitely some neuroendocrine defect which contributes to central neuropathy.5
MATERIALSAND METHODS
This study was undertaken on patients who attended the outpatient departments of General Medicine and ENT of Mahatma Gandhi Medical College and Research Institute, Pondicherry, over a period of one year. The aims of the study were to find out the prevalence and type of hearing loss among diabetic subjects, to establish if there was a relationship between age, duration, severity and complications of Diabetes to the changes in hearing threshold and to establish if there was a relationship between height, weight, family history, diet, blood pressure, blood group and blood cholesterol.The inclusion criteria were: (i) diagnosis of Diabetes mellitus based on National Diabetic Data Group of the National Institute of Health (NDDG) criteria and (ii) cases receiving anti-diabetic treatment showing even a normal range of blood sugar. The exclusion criteria included: (i) history of congenital deafness in the family, (ii) history of head injury and intake of ototoxic drugs, (iii) history of chronic suppurative otitis media, (iv) history of previous ear surgery and (v) history of acoustic trauma and noise induced hearing loss. In this study, 200 age and sex matched healthy subjects formed the control group (Group I) and were evaluated along with 100 diagnosed cases of Non-Insulin Dependent Diabetes Mellitus (NIDDM) without (Group II) and with complications (Group III) such as peripheral neuropathy, non-healing ulcer, diabetic retinopathy, hypertension, nephropathy, congestive cardiac failure or ischaemic heart disease. All patients included in the study were subjected to a thorough systemic and ENT examination to rule out any organic pathology in the external and middle ear. Tuning fork tests were done using 256 Hz, 512 Hz and 1024 Hz tuning forks. All patients were screened for diabetic retinopathy in the retina clinic by direct and indirect opthalmoscopic examination of the fundus. Audiological evaluation was done by Pure Tone Audiometry (PTA) in the Department of ENT using Arphi Digital model 500 MK III audiometer. Both air and bone conduction were tested. Audiograms were recorded for each patient. Patients were examined by a neurologist to rule out peripheral neuropathy, which was confirmed by nerve conduction study. Biochemical estimation of blood urea and serum creatinine was carried out to rule out nephropathy. The patients were categorized into groups according to age, sex, duration, severity and complications of diabetes. Hearing loss was assessed purely based on tuning fork tests and audiometric readings only. Statistical data was analysed using epidemiological information package developed by WHO. To find out the statistical significance, Kruskal-Wallis H test (equivalent to Chi square test) was used since the observations were normally distributed and a large sample was taken. Mean, standard deviation and ‘p’ value were calculated and tests of significance carried out. The bone/air conduction thresholds were measured for both right and left ears in all patients and taken for each frequency 250 to 8000 Hz. The mean was further categorized according to the group and analysed statistically.
RESULTS
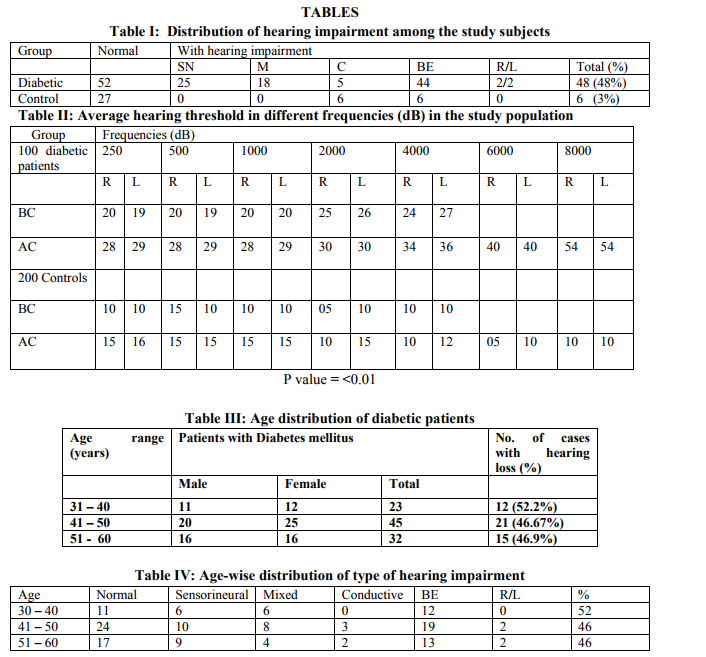
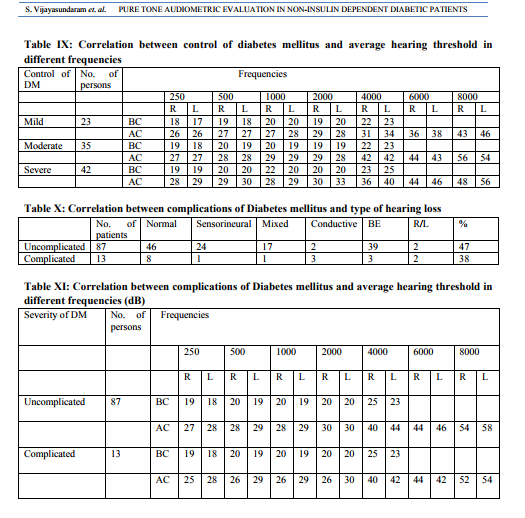
A total of 300 patients were included in the study, of which, 142 were male and 158 were female, with the age ranging from 30 – 59 years. Table I shows the distribution of hearing impairment among the study population. Hearing impairment among the diabetic population is 48% in comparison with non-diabetic population. Diabetes mellitus is the risk factor which contributes four times more risk of hearing loss in comparison with the control population. Table II shows the average hearing threshold for different frequencies for the study population. The mean threshold at each frequency of all control subjects and diabetic patients were calculated, tabulated and compared. The average thresholds in different frequencies were highly significant. The p value is <0.01 both for bone conduction and air conduction in both ears. An average 40% of people were affected by the average threshold in the diabetic group in higher frequencies in both ears, especially in bone conduction. The age distribution of the diabetic cases included in the study is shown in Table III. The age distribution of diabetic patients ranged from 30 to 60 years and included 47 males and 53 females. A statistically significant relationship was established in relation with age and hearing impairment in diabetic patients. Table IV shows the age-wise distribution of the type of hearing impairment while Table V shows the age-wise distribution of average hearing thresholds in different frequencies of the diabetic subjects. 48 out of 100 diabetic patients were found to have hearing impairment, of which, 25 had sensorineural hearing loss, 18 had mixed hearing loss and 5 had conductive hearing loss. Only one ear was affected in 4 patients while both ears were affected in 44 patients. The mean hearing threshold levels in higher frequencies were affected in both ears in diabetic patients. The duration of Diabetes mellitus, type of hearing loss and percentage of hearing loss were analysed and tabulated in Table VI. The number of years as a diabetic significantly affected the average hearing threshold in higher frequencies, especially more in the 10 – 20 years duration group (53.3%), with a P value of <0.05. 8 out of 30 patients in the 10 – 20 years duration group were affected by bilateral high-tone sensorineural hearing loss. The correlation between duration of Diabetes mellitus and the average hearing threshold in different frequencies is shown in Table VII. The mean average threshold in 20 – 30 years duration group in higher frequencies weas increased and statistically significant. The relationship between control of Diabetes mellitus with average hearing threshold and type of hearing loss was tabulated and analysed and is shown in Tables VIII and IX. 25 patients with hearing impairment were found to have poor glycemic control and of these, 12 were found to have sensorineural hearing loss and 13 were found to have mixed hearing loss. The average hearing threshold is calculated by analysis of variance technique. There is marked reduction in hearing threshold from good to moderate in patients with poor control of diabetes, which is highly significant in higher frequencies (P value <0.001 The correlation between complications of Diabetes mellitus and type of hearing loss and average hearing threshold in different frequencies is shown in Table X and Table XI. The patients with no complications had a relatively high threshold in low and high frequencies than those with no complications (speech frequency equal). There was no statistical significant relationship found.
DISCUSSION
In this study, the average hearing thresholds for higher frequencies of 40% of patients in the diabetic group were found to be affected, especially in bone conduction. Similar results were seen in studies carried out by Axelssonet al3 , Taylor et al7 , Costa19, Kurien et al11, Kutty25 , Agarwal1 and Sharma23 . A positive relationship between age and hearing impairment among diabetics was reported by Kutty25, Agarwal1 and Sharma23. In our study, 48% of diabetic patients were found to have hearing impairment and these patients fell in the 40 – 60 years age group, most having sensorineural or mixed type of hearing loss, with both ears being affected in the majority. A positive correlation between duration of diabetes mellitus and hearing loss was reported by Zelenka et al6 , Axelsson et al3 , Tay26 and Sharma23.Kurien et al11 and Cullen et al12 and few other studies20,21,22,27,28 did not find such a relationship. Duration of diabetes mellitus significantly affected the average hearing threshold in higher frequencies, which was found to be especially significant in the 10 – 20 years duration group. Our study showed a marked reduction in hearing thresholds from good to moderate in patients with poor control of diabetes, which was highly significant in higher frequencies. A similar relationship was reported by Agarwal1 and Sharma24.Krocks collaboration study group (1984)13 and other studies14-18 stated that good control of Diabetes mellitus would decrease or postpone the angiopathic changes in the end organs. Axelssonet al4 , Tay26 and Kutty25 found no relationship between severity of the disease and the hearing threshold.Jorgenson5 , Kurien11 , Agarwal1 , Sharma23,24 showed a distinct relationship between diabetic retinopathy, neuropathy and microangiopathy and the hearing threshold. Our study showed no statistical significant relationship between complications of diabetes mellitus and type of hearing loss and average hearing threshold in different frequencies.
CONCLUSION
The study showed that diabetics have an increased mean threshold at higher frequencies in comparison to non-diabetics. Older age groups, longer duration of diabetes and poor control of diabetes showed strong correlation with significant bilateral high frequency hearing impairment as compared to presbyacusis. No statistically significant relationship was found between hearing impairment and complications of diabetes, weight or other parameters.
CONFLICT(S) OF INTEREST:
NONE ACKNOWLEDGEMENT
The authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/ editors/ publishers of all those articles, journals and books from where the literature for the article has been reviewed and discussed.

References:
REFERENCES
1. Agarwal MK. Otorhinolaryngological studies in diabetics. IJO and HNS 1998;50:116-121.
2. Verma A, Bisht MS, Ahuja GK. Involvement of central nervous system in diabetes mellitus. Journal of Neurology, Neurosurgery and Psychiatry 1984;47:414-416.
3. Axelsson A, Fagerberg SE. Auditory function in diabetics. ActaOtolaryngologica 1968;66:49-64.
4. Axelsson A, Fagerberg SE. Hearing in diabetics. ActaOtolaryngologica 1978;356:1- 23 (supplement).
5. Jorgensen MD, Buch NH. Studies on inner ear functions and cranial nerves in diabetics. ActaOtolaryngologica 1961;53:350-364.
6. Zelenka J, Kozak P. Disorder in the bloodsupply of the inner ear as an early symptom of diabetic angiopathy. JLO 1965;79:314-319.
7. Taylor IG, Irwin J. Some audiological aspects of diabetes mellitus. JLO 1978;92:9-13.
8. Gibbin KP, Davis CG. A hearing survey in diabetes mellitus. Clinical Otolaryngology 1981;6(3):345-350.
9. Robin PE. Deafness and Diabetes (Editorial). Clinical Otology 1981;6:309.
10. Miller JJ, Beck L, Davis A, Jones DE, Thomas AE. Hearing loss in patients with diabetic retinopathy. AJO 1983;4:342-346.
11. Kurien M, Thomas K, Bhanu TS. Hearing thresholds in patients with diabetes mellitus. JLO 1989;103:164-168.
12. Cullen JR, Cinnamond MJ. Hearing loss in diabetics. JLO 1993;107;179-182.
13. KrocsColloboration Study Group. Blood glucose control and the evolution of diabetic retinopathy and albuminuria. NEJM 1984;311:365-376.
14. Olsen S, Noffsinger D. Comparison of one new and three old tests of auditory adaptation. Archives of Otolaryngology 1974;99:94-99.
15. Friedman SA, Schulman RH, Weiss S. Hearing and diabetic neuropathy. Archives of Internal Medicine 1975;135:573-576.
16. Sieger A, White NH,Skinner MW, Spector GJ. Auditory function in children with diabetes mellitus. Annals of Otology, Rhinology and Laryngology 1983;92:237-241.
17. Wilson R, Soeldner JS. The relationship of idiopathic sudden hearing loss to diabetes mellitus. Laryngoscope 1982;92:155-160.
18. Snashall SE. Bakesey audiometry and tone and reflex decay tests in diabetes. Archives of Otolaryngology 1977; 103:342-343.
19. Costa OA. Inner ear pathology in experimental diabetes. Laryngoscope 1967;77:68-75.
20. Virtaniemi J, Laakso M, Nuutien J. Auditory brainstem latencies in type I (IDDM) diabetic patients. AJO 1993;14(6):413-418.
21. Rudolph. Inner ear damage secondary to Diabetes mellitus. Arch Otolaryngol Head Neck Surg 1990;117:635-640.
22. Wackym PA, Linthicum FH. Diabetes mellitus and hearing loss: clinical and histopathological relationship. AJO 1986;7:176-182.
23. Sharma R et al. Brain stem evoked response in patients with diabetes mellitus. IJO 2000;52:223-228.
24. Sharma R. Audiovestibular changes in diabetes mellitus. IJO and HNS 1999;51:40-43.
25. Kutty SR et al. Hearing loss in diabetes mellitus. IJO and HNS 1998;4:131-135.
26. Tay HL, et al. Diabetes mellitus and hearing loss. ClinOtolaryngol 1995;20(2):130-4.
27. Huang YM, Pan CY, Rui G, Cai XH, Yu LM, Chou CY. Study on the hearing impairment in diabetic patients. Chinese Journal of Otorhinolaryngology 1990;25:354-356.
28. Carmen RE, Svihovec DA, Gocka EF, Gay GC, House LR. Audiometric configuration as a reflection of low plasma glucose and diabetes. AJO 1989;10:372-379.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License