IJCRR - 6(10), May, 2014
Pages: 112-119
Print Article
Download XML Download PDF
DYNAMIC BACTERIAL PROFILE OF ENDOTRACHEAL ASPIRATES AND ITS SENSITIVITY PATTERN -A CAUSE OF CONCERN
Author: N. Shanmuga Vadivoo, Priya Santharam, K. Sudha, G. Kalaiselvi, B.K. Padmavathi, B. Usha, Amar Kumar, Nitesh Kumar Jaiswal
Category: Healthcare
Abstract:Aim: To analyse the changing spectrum of aerobic bacteria isolated from endotracheal secretions of Mechanically ventilated patients andto evaluate the antibiotic sensitivity pattern & Multi drug resistance of those isolates Materials and Methods: Endotracheal secretions received during the study period from Oct 2010 to Dec 2012 were processed & all the pathogenic isolates were identified as per the standard guideline. Antibiotic sensitivity was performed for the identified pathogens according to CLSI standards. Clinical condition of the ventilated & tracheotomised patients were recorded. Results: A total of 95 Endotracheal isolates were processed and 73 % of the aspirates were showing growth. The incidence of VAP was 33.3% and most frequently isolated pathogens were Klebsiella spp(36%), Pseudomonas(17%) &Acinetobacter spp(18%).Multidrug resistance was observed in 53% of Klebsiella spp, 26% in Pseudomonas and 56% in Acinetobacter spp.Most of the patients (33%)were ventilated for Trauma and hence most of the patients had prolonged hospital stay \?average time being 25 days. Conclusion: In this study we observed that there was a gradual shift in the isolated aerobic bacterial profile. Initially in 2011 the frequent pathogen was Klebsiella spp(41%) &Pseudomonas spp(18%) when Acinetobacter was 10% . In 2012 Acinetobacter was found to be the most prevalent pathogen (30%) and klebsiella spp prevalence reduced to 28%. Multidrug resistant was also observed among the isolated pathogens. Prolonged hospital stay for trauma has resulted in multidrug resistance and change in bacterial profile. Empirical antibiotic therapy was recommended based on the antibiogram of the most prevalent pathogen in ICU.
Keywords: Dynamic, Bacterial profile, Endotracheal aspirates
Full Text:
INTRODUCTION
Uses of invasive drugs and therapeutic methods have saved many lives but on the other hand it has caused life threatening consequences due to severe persistent resistant infections [1]. Hence following these invasive therapeutic and diagnostic methods the incidences of nosocomial infections particularly in ICU’s and CCU’s have raised[2, 3, 4, 5]. There is much well documented evidence that hospital personnel and environment are the microbial source and prolonged hospital stay& overuse of antimicrobial agents has led to multidrug resistance of these microbes [6] . Intensive care patients on Mechanical ventilation/ orotracheal intubation arefrequently colonized with this microbial sourceof exogenous origin orendogenously from patients themselves [7, 8, 9] . These colonized bacteria cause eitherVentilation
associated Tracheo bronchitis(VAT)orVentilated associated Pneumonia (VAP)[10, 11] . Colonizationmay be caused by a wide spectrum of bacterial pathogens, which may be polymicrobial.Common pathogens include aerobic gram-negative bacilli, such as Pseudomonas aeruginosa, Escherichia coli, Klebsiellapneumoniae and Acinetobacterspecies[11- 14]Infections due to Gram-positive Cocci, such as Staphylococcus aureus, are more common in patients with diabetes mellitus and head trauma.The colonization of the enteric GNB is frequently observed in patients who have prolonged hospital stay as in the case of Trauma/head injury. As the traumatic patients stay longer, the type of infecting flora change in due course of therapy [15] Despite advances in patient care, these changing floras complicate therapy by acquiring drug resistance and altering their sensitivity pattern [16].Therefore updated knowledge of local epidemiological and susceptibility profile is recommended for guiding the clinicians regarding empirical choice of antibioticsand has become mandatory along with adequate clinical diagnosis and bacterial confirmation [17]Hence the aim and objective of the study is to retrospectivelyanalyze the spectrum of aerobic bacteria isolated from endotracheal aspirates of tracheostamised patients & Patients on Mechanical ventilation, to evaluate the antibiotic sensitivity pattern & Multi drug resistance of those isolates, to record the demographic details, Clinical diagnosis for which the patient was intubated, length of ICU stay and duration of ventilation
MATERIAL AND METHODS
The present retrospective study was conducted at the Microbiology Department of a teaching tertiary care hospital over a period of two years and three months (October 2010 – December 2012). Endotracheal secretions (ETS) were collected aseptically from the patients of all age groups and sex admitted to intensive care unit, who were on mechanical ventilation for at least 3 days and with clinical suspicion of VAP or VAT.All the specimens received were immediately plated on the blood agar and MacConkey agar by semi quantitativemethod and incubated aerobically overnight at 37°C. Organisms were identified as commensal or pathogen as per protocol. Single or mixed growth (two or more than two isolates per specimen) isolated from all the eligible single and consecutive samples were identified by observing the colony characteristic on the blood, MacConkey agar plate, and biochemical reactions using standard microbiological methods. Isolates from repeat culture of previously recruited patients and isolates identified as commensal or contaminants were excluded. The following antibiotics (Hi-Media Disc in μg) were tested for Gram negative bacilli: Ampicillin10(A), Piperacillin-100(Pi), Ceftazidime30(Ceftaz), Ceftazidime+clavulinic acid-30/10 (CaC), Cefaperazone-30(CPZ), Cefotaxime30(CTX), Cefotaxime +clavulinicacid30/10(CxC), Ceftriaxone-30(CFTR), Ampicillin/Sulbactum-10/10(AmpS), Piperacillin/tazobactum-100/10(PiT), Aztreonam30(Ao),Gentamicin -10(Gen), Amikacin-30 (Ak), Ciprofloxacin-5(Cip), Ofloxacin-5(Ofl), Imipenem-10(IPM) and Colistin-10(CL).Zone diameter was measuredand interpreted as per the Clinical and Laboratory Standards Institute guidelines.ESBL production was also detected by Phenotypic confirmatory method as per CLSI Guideline
RESULTS
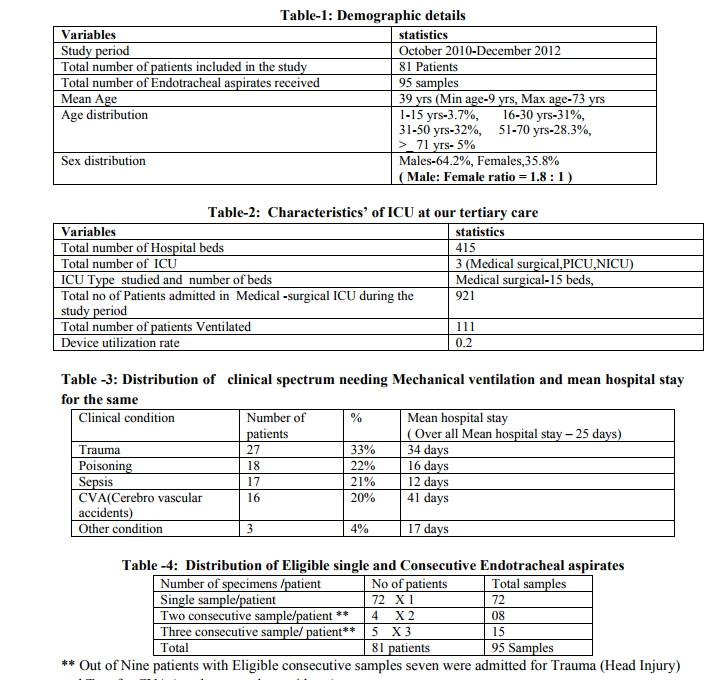
During the study period of two years and three months (Oct 2010 – Dec 2012), laboratory data of 95 Endo tracheal aspirates specimens from 81 patients received in our laboratory were evaluated. The demographic data’sand Characteristics’ of ICU are described in Table 1 & Table-2.The most
frequent clinical condition needing mechanical ventilation was for Trauma(head injury) and the mean hospital stay was 25 days .The clinical conditions for mechanical ventilation and the mean hospital stay for each condition are discussed in Table-3. From 72 of the 81 patients (89%), single sample was received and from nine patients (11%) consecutive samples were received (Table- 4). Sixty six of 81 patients (82%) showed bacterial growth in their ETS. Out of 95 endotracheal aspirates received, 70specimens (73 %) were culture positive, whereas 25 (27%) specimens showed no growth. Eighty nine (89) bacterial isolates were recovered from 95 endotracheal aspirates and 18.6% of endotracheal aspirates had polymicrobial growth.Bacterial profile of 89 isolates is shown in Table-5.Out of the total 89 isolates identified, 92% were gram-negative bacilli (GNB) and 8% were gram-positive organisms. Aerobic gram negative bacilli remained the predominant pathogens from mechanically ventilated patients throughout the study period. Frequency of changing bacterial profile of the three prevalent pathogens and its sensitivity pattern are shown in Table-6 and from Table-7 to Table-9.
DISCUSSION
Health care associated infections (HCAI) continue to be a major cause of patient morbidity and attributable mortality and device associated infections contributes a maximum towards HCAI.The mechanically ventilated and Tracheostamized patients are colonized with bacteria of either endogenous or exogenous origin which might end up in VAT or VAP.The incidence of VAP at our center during the study period was 33.3% with Device utilization rate being 0.21. As mentioned before changing bacterial profile from ETS and its sensitivity pattern are analyzed and discussed below. Our study showed 73% bacterial growth from ETS received at our Microbiology lab. The percentage is less when compared to following studies which show bacterial growth of 92% [17], 90%[18], and 78% [19] but higher when compared to a study by Shalini S etal[20]where 67.3% of ETS showed growth . In our study the most frequent clinical conditions needing mechanical ventilation was Trauma (Head injury) accounting to 33 % followed by Poisoning at 22% as shown in Table-3. In a study by Arindam de Post operative condition was the most frequent clinical condition needing mechanical ventilation[13] and COPD patients were the most frequently ventilated patients in a study by Ramakrishna pai[14]. The mean hospital stay for Head Injury patients were 34 days and for poisoning 16 days. But the maximum mean hospital stay was for CVA with 41 days. Table -4 shows that 73% of (72/95) ETS was a single sample whilethe rest 27 % (23 /95) were consecutive sample. The eligible consecutive samples were from Head trauma and CVA patients who had more than a month of mean hospital stay. Bacterial growth was seen in 91% of Trauma/Head Injury patients followed by 83% of CVA patients. And as indicated before these patients had more than 30 days of ICU stay .This correlates with the fact that Head injury leads to prolonged hospital stay which ultimately results not only in increased bacterial growth rate but also changing bacterial spectrum of ETS [15] Among the bacterial profile of 89 isolates (Table5) Gram negative Bacilli were 92% among the 89 isolates and only 8% were Gram positive cocci.This result is supported by many studies and particularly as shown in one systematic review article where GNB’s range from 41-92% and Gram positives between 6-58%[21]. The most predominant pathogen of ETS during the two year study period was Klebsiellaat 35.9% followed by Acinetobacter spp at 17.9% and Pseudomonas at 16.8% (Table-5). This is in accordance with other
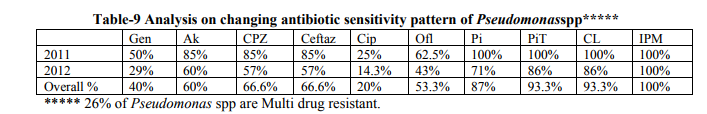
studies where in Klebsiella is the most prevalent one at 34% [14] and 38.4%[20] . Acinetobacter was the predominant in the following studies [3, 7, 13, 17, 19, 22]and Pseudomonas was 40.3 % in a study by Koirala.P[18] . E.coli (33.3%) was the predominant in a study by Tullaet al [12] .Gram positives were the predominant in the following study by Maryam etal [23]. In the study even though Staphylococcusaureus was the prevalent one, among the gram negatives Klebsiella(23.3%) was the foremost one followed by Acinetobacterspp at 20% which is similar to our study. Our review demonstrates that there is a change in microbial spectrum during the study period as in Table-6, which shows dynamic bacterial profile of the three most prevalent pathogens.Klebsiella species was the most prevalent one during 2011 at 41% followed by Pseudomonas 18% and Acinetobacter species at 10.2%.This profile altered during 2012, where in Acinetobacter prevalence rate increased to 30.1% followed by Klebsiella at 28.2% and Pseudomonas spp at 17%.This changing bacterial profile at our hospital could be attributed to Prolonged hospital stay among the study group patients .The changing bacterial profile of ETS was also described in a study by Maryam amini[23] . We reviewed not only the changing bacterial profile but also their sensitivity pattern during the study period. Similar to our study the changing antibiotic sensitivity pattern was also analyzed and discussed in a study by S.Kaulet al [16] .Analysis on changing sensitivity pattern of the three most prevalent pathogens of ETSKlebsiella spp, Acinetobacter spp & Pseudomonas sppat our tertiary care center is shown in Table-7, Table-8 and Table-9 respectively Analysis on changing sensitivity pattern of Klebsiella spp shows that there was an increase in percentage sensitivity for Imipenam, ciprofloxacin &Ceftazidime in 2012 when compared to 2011. Sensitivity towards Aminoglycosides &Cefotaxime decreased in 2012. There was relatively overall good sensitivity percentage (i.e.) more than 50% sensitivity forAmikacin (56.2%),Ofloxacin(64%) and Imepenam(72%). Other antibiotics showed less than 30% sensitivity with ciprofloxacin at 28%, Cefotaxime at 25% and Gentamicin at 28%.Multi drug resistance to Klebsiella at our hospital setting was at 53% and ESBL producing Klebsiella were 85%. Ina study by Santosh kanal et al [19], MDR to Klebsiellawas at 73% where in Klebsiella was 31% sensitive to Gentamicin, 21% to Cefotaxime and 45% to Ciprofloxacin. In a study by Mehta et al[2] Imepenam sensitivity to Klebsiellawas alarmingly low at 50%! In our tertiary care centre Acinetobacter species showedoverall good sensitivity to Imipenam at 94%, but there was a decrease in percentage sensitivity when compared to 2011. Aminoglycoside sensitivity drastically decreased in the year 2012 when compared to 2011 as shown in Table-8. There was an increase in sensitivity to Ciprofloxacin from 25% in 2011 to 33.3% in 2012. Piperecillin-Tazobactum sensitivity almost remained same during the study period at 70%.Multi drug resistance to Acinetobacter at our center was 56 % which is low when compared to following studies by Santosh khanal [19] and Werarek.P [22], where Acinetobacter was 85% & 92% MDR respectively.ESBL producing Acinetobacter was 80% Third most Prevalent pathogen Pseudomonas had overall very good sensitivity to many antimicrobial agents except Ciprofloxacin. Imipenam sensitivity was consistently at 100% throughout the study period. The other antibiotics started to show a decrease in sensitivity percentage as shown in Table-9. MDR percentage of Pseudomonas at our centre was 27% which is low when compared to other studies [19].Ina study by Koirala et al [18] , Pseudomonas had Zero percent sensitivity to Ceftazidime, Amikacin at 22% and Gentamicin at 18%. Sensitivity to ciprofl0oxacin was at 19% which is similar to our study.
CONCLUSION
The frequency of specific MDR pathogens colonizing upper respiratory tract and causing Tracheobronchitis or VAP’s may vary by hospital, with in hospital in different units, type of ICU patient, patient population, exposure to antibiotics, and changes over time. Hence periodic surveillance to identify MDR pathogens and their antibiotic sensitivity pattern will allow appropriate empirical antibiotics followed by earlier targeted antibiotic treatment which could improve outcome in patients and prevent VAP. This emphasizes the need for timely periodic local surveillance data .Considering these important factors in to account, this Review has addressed the changing bacterial profile, changing AST pattern of most prevalent pathogens over the time &multidrug resistance among the three most prevalent pathogens isolated from Endotracheal aspirates at our center. Gram negatives were the predominant pathogens. High rate of resistance to Cephalosporin, Fluoroquionolone and Amino glycosides wasnoted for all the three prevalent pathogen. ButImipenam resistance was seen only in Klebsiella&Acinetobacter sppand not in Pseudomonas. Following this retrospective study, pipericillin-tazoactum, ampicillin-sulbactum, and imipenam were proposed as empirical antimicrobial choice for patients diagnosed with clinical VAP or VAT at our center. These empirical antibiotics were recommended to be given alone or in combination depending on severity of Patient’s clinical condition and renal parameters.Also recommendations were made such that antibiotic therapy should be changed and deescalated based on Culture identification report and a specific antibiotic should be chosen based on sensitivity pattern.An Active Surveillance for VAP was also initiated as a measure of Hospital Infection control at our tertiary care centre.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.

References:
REFERENCES
1. Victor D. Rosenthal et al.Device-Associated Nosocomial Infections in 55 Intensive Care Units of 8 Developing Countries.Ann Intern Med. 2006; 145(8):582-591.
2. A. Mehta a, V.D. Rosenthal et al.Deviceassociated nosocomial infection rates in intensive care units of seven Indian cities. Findings of the International Nosocomial Infection Control Consortium (INICC). Journal of Hospital Infection (2007) 67, 168- 174.
3. SS Kanj, ZA Kanafani, N Sidani, L Alamuddin, N Zahreddine, and VD Rosenthal. International Nosocomial Infection Control Consortium Findings of DeviceAssociated Infections Rate in an Intensive Care Unit of a Lebanese University Hospital. J Glob Infect Dis. 2012 Jan-Mar; 4(1): 15–21.
4. SALOMAO, Reinaldo et al. Device-associated infection rates in intensive care units of Brazilian hospitals: findings of the International Nosocomial Infection Control Consortium. Rev PanamSaludPublica [online]. 2008, vol.24, n.3, pp. 195-202.
5. http://www.who.int/csr/resources/publications/ drugresist/en/whocdscsreph200212.pdf 6. Mukhopadhyay C, Bhargava A, Ayyagari A. Role of mechanical ventilation and development of multidrug resistant organisms in hospital acquired pneumonia. Indian J Med Res 2003; 118:229-35.
7. Joseph NM, Sistla S, Dutta TK, Badhe AS, Parija SC. Ventilator-associated pneumonia: role of colonizers and value of routine endotracheal aspirate cultures.Int J Infect Dis. 2010; 14(8):e723-9.
8. T.J.J.InglisE,W.Lim G,,.H.Lee,Cheong NG. Endogenous source of bacteria in tracheal tube and proximal ventilator breathing system in intensive care patients.British journal of Anaesthesia 1998; 80:41-45.
9. Fagon JY, Chastre J, Hance AJ, Montravers P, Novara.A, Gibert C. Nosocomial pneumonia in ventilated patients: A cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993; 94:281–8. [Pub Med: 8452152]
10. Niederman MS, Ferranti RD, Zeigler A and Reynolds HY () Respiratory infection complicating long-term tracheostomy. The implication of persistent gramnegative tracheobronchial colonization. Chest 1984; 85(1): 39-44
11. Donald E. Craven and Karin I. Hjalmarson. Ventilator-Associated Tracheobronchitis and Pneumonia: Thinking Outside the Box. Clinical Infectious Diseases 2010; 51(S1):S59–S66. 12. MS Tullu, CT Deshmukh, SM Baveja .acterial profile and antimicrobial susceptibility pattern in catheter related nosocomial infections..JPGM, 1998: 44 : 1 : 7-13.
13. ArindamDey , Indira Bairy.Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: A nine months' prospective study. AnnThorac Med. 2007 Apr-Jun; 2 (2): 52–57.
14. Ramakrishna PaiJakribettu and RekhaBoloor.Characterisation of aerobic bacteria isolated from endotracheal aspirate in adult patients suspected ventilator associated pneumonia in a tertiary care center in Mangalore.Saudi J Anaesth. 2012 Apr-Jun; 6(2): 115–119.
15. Gotsman MS and Whitby JL .Respiratory infection following tracheostomy. Thorax 1964; 19:89-96
16. S Kaul, KN Brahmadathan, M Jagannati, TD Sudarsanam, K Pitchamuthu, OC Abraham.One year trends in the gramnegative bacterial antibiotic susceptibility patterns in a medical intensive care unit in South India.. Indian Journal of Medical Microbiology, (2007) 25 (3):230-5.
17. JoãoManoel da Silva Júnior, EderlonRezendeetal .Epidemiological and Microbiological Analysis of VentilatorAssociated Pneumonia Patients in a Public Teaching Hospital. BJID2007; 11(5):482-488.
18. PratirodhKoirala, Dwij Raj Bhatta, PrakashGhimire, Bharat Mani Pokhrel, UpendraDevkota.Bacteriological Profile of Tracheal Aspirates of the Patients Attending a Neuro-hospital of Nepal. Int J Life Sci 4:60- 65.
19. SantoshKhanal,Dev Raj Joshi, Dwij Raj Bhatta, UpendraDevkota,and Bharat Mani Pokhrel. β-Lactamase-Producing MultidrugResistant Bacterial Pathogens from Tracheal Aspirates of Intensive Care Unit Patients at National Institute of Neurological and Allied Sciences, Nepal. ISRN Microbiology.Volume 2013. Article ID 847569, 5 pages.
20. Shalini s, Kranthi k, Gopalkrishnabhat k. The Microbiological Profile of Nosocomial Infections in the Intensive Care Unit. Journal of Clinical and Diagnostic Research. 2010 October ;(4):3109-3112.
21. YaseenArabi a, Nehad Al-Shirawi a, ZiadMemish b, Antonio Anzueto. Ventilatorassociated pneumonia in adults in developing countries: a systematic review. International Journal of Infectious Diseases (2008) 12, 505—512.
22. Werarak P, Kiratisin P, Thamlikitkul V. Hospital-acquired pneumonia and ventilatorassociated pneumonia in adults at SirirajHospital: etiology, clinical outcomes, and impact of antimicrobial resistance. Med Assoc Thai. 2010 Jan; 93 Suppl 1:S126-38.
23. Maryam Amini , Ahmad Javanmard, Ali Davati ,GhasemAzimi . Bacterial Colonization in Tracheal Tubes of ICU Patients. Iranian journal of pathology. 2009; 4: (3).123-127.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License