IJCRR - 9(2), January, 2017
Pages: 14-19
Date of Publication: 04-Mar-2017
Print Article
Download XML Download PDF
The Prevalence of Philadelphia Chromosome in BCR-ABL Positive Chronic Myelogenous Leukemia Cases in North Indian Population
Author: Fatima Bhopalwala Ali1, R.K. Verma2, Mustafa Ali3, Navneet Kumar4
Category: Healthcare
Abstract:Aim: The aim of my study was to identify the prevalence of Philadelphia chromosome in BCR-ABL positive CML cases in North Indian population. Philadelphia (Ph) chromosome is a specific cytogenetic marker, essentially required for the diagnosis, clinical management and follow up of patients of chronic myelogenous leukemia (CML), which is a myeloproliferative neoplasm of hematopoietic cell. It results from a reciprocal translocation, t (9; 22) (q34; q11.2) in the hematopoietic stem cells. The molecular consequence is the formation of the chimeric BCR ABL1 gene which plays a central role in the development of CML.
Methods: Bone marrow and peripheral blood sample from 66 CML cases were collected from Dept. of Clinical Hematology, KGMU. The samples culture and cytogenetic analysis was done in the Dept. of Anatomy, KGMU.
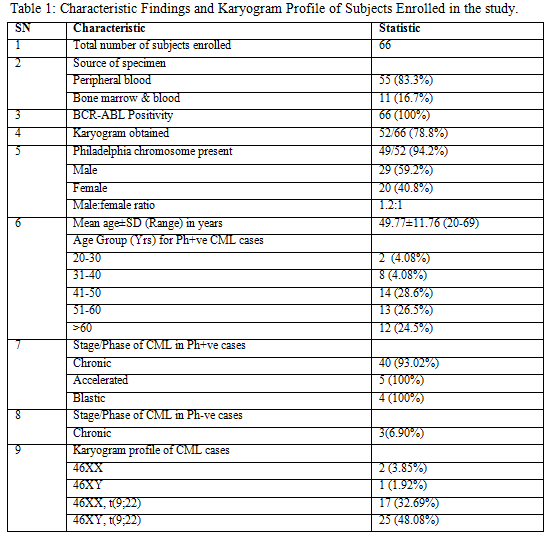
Results: Out of the total 66 cases, karyograms of 52 cases (78.8%) were obtained. 49 cases (94.2%) showed the presence of Philadelphia chromosome, while 5.8% cases showed normal karyogram. Most common age group showing the presence of Ph+ve CML was 41-50 years (28.6%) followed by patients in 51-60 years group. Majority of cases were males (59.2%). Compared with stage of CML 81.6% of Ph+ve cases were in chronic phase.
Conclusion: Majority of the CML cases were Philadelphia positive, with males more commonly involved. Ph+ve CML was seen commonly above 40 years of age with maximum patients presenting in chronic phase of CML. Our results are at par with research reports published around the world on the Philadelphia chromosome prevalence in CML.
Keywords: Chronic myelogenous leukemia, Philadelphia chromosome, BCR-ABL
Full Text:
INTRODUCTION
The cytogenetic hallmark of CML is the Philadelphia (Ph) chromosome that results from a reciprocal translocation between chromosome 9 and 22,
i.e. t (9; 22) (q34; q11.2)1, occurring in the hematopoietic stem cells. The reciprocal translocation in this CML results in the fusion of breakpoint cluster region (BCR) gene on chr. 22q11 with the ABL1 (named after the abelson murine leukemia virus) gene located on chromosome 9q34. Thus, the molecular consequence of this translocation is the formation of the chimeric BCR-ABL gene, usually located on the Philadelphia chromosome1. BCR-ABL plays a central role in the development of CML. The BCR-ABL chimeric gene is transcribed into BCR-ABL1 mRNA, which in turn encodes a fusion protein p210BCR-ABL1. Rarely a breakpoint occurring in BCR gene may produce another fusion protein p230BCR-ABL1. The exact mechanism(s) by which these fusion genes promote the transition from benign to fully malignant state is still unclear. The attachment of BCR sequence to ABL results in three critical changes viz.
1) The ABL protein becomes constitutively active as a tyrosine kinase enzyme, subsequently activating downstream kinases that prevent apoptosis;
2) The DNA-protein-binding activity of ABL is attenuated;
3) The binding of ABL to cytoskeleton actin microfilaments is enhanced1, 2.
Imatinib mesylate (Glivec, formerly STI571) is a rationally developed, orally administered inhibitor of the BCR-ABL protein. Recent karyotype analyses show that 60%–70% of patients achieve complete disappearance of Ph-positive marrow cells and maintain exclusively Ph-negative bone marrow cells (a state designated as a complete cytogenetic response [CCyR]) 5 years after initiating imatinib treatment3 and overall survival is between 80-95%4. The gold standard for the detection of chromosomal aberrations is karyotyping5. Till date most of the work regarding the cytogenetics of CML and its significance in the diagnosis, prognosis, and treatment of the disease has been reported from countries other than India (Hehlmann et al., 2007; Fabarius et al., 2011)6,7, while such studies reported from our region are limited (Chavan et al., 2006; Anand et al., 2012).8,9 Thus, the present study was done to determine the prevalence of Philadelphia chromosome in BCR-ABL positive individuals where the BCR-ABL positivity was confirmed by Fluorescent-In-Situ-Hybridization (FISH) assay prior to enrollment.
MATERIAL AND METHODS
Selection criteria and Collection of samples-
The study was conducted after obtaining the approval from the Ethical Committee of the King George's Medical University U.P., Lucknow. Screening of the patients was done in the Department of Clinical Hematology, and samples were collected in the hematology laboratory of the same department and also in the Department of Pathology, King George Medical University U.P., Lucknow. The consent was taken from each participant after explaining the purpose of the study. The diagnosed cases of CML (diagnosis confirmed on the basis of clinical and hematological evaluation) irrespective of age and sex were included in the study. Lack of confirmed diagnosis and/or consent from the patient served as the exclusion criteria. The CML cases were BCR-ABL positive as detected by Fluorescent-In-Situ-Hybridization (FISH) assay prior to enrollment. Bone marrow aspirate and/or peripheral blood samples of the CML patients were collected. Detailed personal history, occupational history was taken and thorough clinical examination was done at time of sample collection.
Preparation of Karyogram
Harvesting of sample
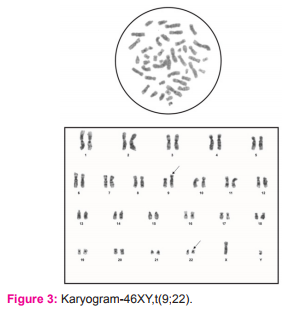
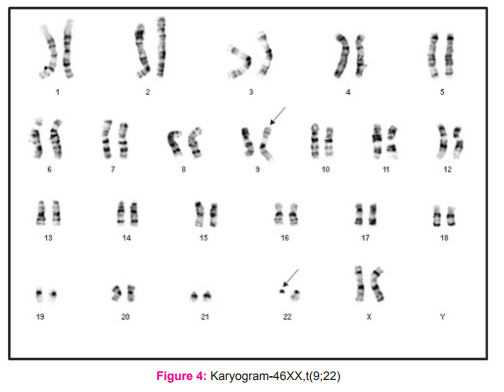
Bone marrow aspirate and blood sample of the CML patients were collected in BD Vacutainer sodium heparin vial. The sample was taken in a test tube containing culture media (RPMI 1640) and incubated in CO? incubator in slanting position. After incubation, Colchicin solution was added and test tube was again incubated for one hour and then centrifuged at 1000 rpm for 10 minutes. Supernatant was discarded by pipetting of media leaving as little medium as possible over the cell button at bottom of test tube. Cell button was suspended in hypotonic solution (Potassium chloride + Sodium citrate). Slides were prepared by dropping method, and were treated with trypsin to obtain better banding. Adequately aged slides were stained with Giemsa stain. Karyotyping results were obtained by analyzing 20 metaphase fields for each case and in cases where abnormal karyotype were suspected, the observation was extended to a total of 30 fields. The karyotypes were reported as per International System for Human Cytogenetic Nomenclature (ISCN, 2013) guidelines.10
Statistical analysis
Data was analyzed using Statistical Package for Social Sciences (SPSS) version 15.0. Data has been represented as frequencies and percentages and mean and standard deviation. Chi-square test has been used for the purpose of analysis. The confidence level of the study was kept at 95%, hence a “p” value less than 0.05 indicated a significant association.
RESULTS
A total of 66 CML patients were enrolled out of which 52/66 (78.8%) karyograms were found satisfactory. Thus these 52 karyograms of BCR-ABL positive hematologically confirmed cases of Chronic Myelogenous Leukemia were analyzed. The CML cases in the present study comprised of adult patients with their ages ranging between 20- 69 years and the mean age was 49.77 years (table 1). Majority of patients were male (n=29; 59.2%). There were 20 (40.8%) females (table 1). Male to female ratio of study population was 1.36:1.
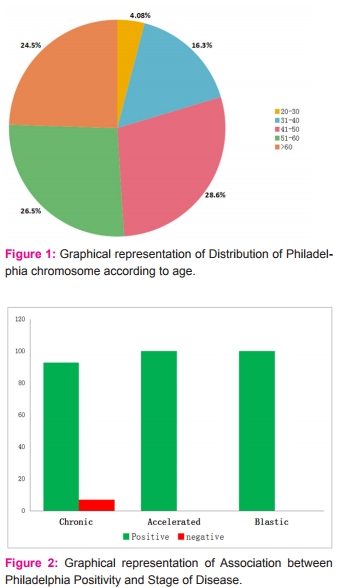
In our study all the patients had BCR-ABL analysis done at the time of diagnosis of CML and the BCR-ABL positivity was 100%, while on cytogenetic analysis done on these patients Philadelphia chromosome was seen in 49 out of 52 cases, and rest 3 cases showed a normal karyogram without any translocation i.e.; Ph negative BCR-ABL positive CML cases (5.8%). The Ph+ve BCR-ABL+ve CML cases were 94.2 % (figure 2). The association between the presence of Philadelphia chromosome in a patient and the clinical stage of CML is shown in the figure 2. The majority of Philadelphia positive (93.02%) and all the Philadelphia negative patients were in chronic phase of CML. Accelerated phase CML was seen in 5 Philadelphia positive cases and blast phase in 4 of Ph+ve cases only. However, association between Philadelphia status and stage of disease was not statistically significant (p=0.717).
DISCUSSION
Conventional cytogenetic analysis has been considered mandatory for all newly diagnosed cases of leukemias, because karyotyping plays a vital role in their diagnosis, classification and prognostification11. This holds true for chronic myelogenous leukemia as well. The frequency of CML has been increasing worldwide and so the disease burden on the diagnosis and treatment aspect as well is increasing. This has opened a wide area of potential research for the medical fraternity in the field of cytogenetics of chronic myelogenous leukemia.
Our study group consisted of 66 cases of CML who were hematologically confirmed, and their consent was obtained prior to enrollment. For all leukemia types, the age adjusted incidence is found to be higher in males, with a ratio of 1.9 vs. 1.1 (male vs. female) in case of chronic myelogenous leukemia 5. Similarly, the male patients included in the present study were 36 (54.5%) and females were 30 (45.5%) and thus CML was found to be more common in men, (Figure 3) with a male: female ratio of 1.2:1 among the total 66 cases of CML enrolled (Table 1). Our findings were in resonance with the study conducted by Chavan et al8 who found a male: female ratio 1.9:1 out of 175 haematologically confirmed CML cases. The CML cases in the present study comprised of adult patients with their ages ranging between 20- 69 years and the mean age was 49.77 years (Table 1). Our results regarding age of patients were same as seen by Boronova et al12 who analyzed 72 CML patients and found their age in the range of 19-74 years with median age of 46.4 years (Presov region, Slovakia). The hallmark of CML is Philadelphia chromosome, which causes formation of chimeric BCR-ABL oncogene at the molecular level. In our study all the patients had BCR-ABL\ analysis done at the time of diagnosis of CML and the BCR-ABL positivity was 100%, while on cytogenetic analysis done on these patients Philadelphia chromosome was seen in 49 out of 52 cases, and rest 3 cases showed a normal karyogram without any translocation i.e.; Ph negative BCR-ABL positive CML cases (5.8%). The Ph+ve BCR-ABL+ve CML cases were 94.2% (figure 2). Our findings were in agreement with another Indian study of Anand et al, 9 who found 96% cases to be Ph positive at the cytogenetic level. All of the Ph negative BCR-ABL positive patients were in CML chronic phase. Also, Boronova et al12has detected Philadelphia chromosome in 94.4% of CML cases in Presov region of Slovakia. This comparison signifies that presence of Philadelphia chr. in CML cases is uniform and comparable in different regions of the world.
It has been well documented in the past that few CML patients may not demonstrate Ph chromosome (Ph chromosome negative and BCR-ABL positive), yet have clinical course and morphological features just like typical CML4. We experienced the same in the present study where no difference in the presentation was seen between Philadelphia positive and negative CML subjects.
The prognosis for patients with Ph+ve CML than those without this translocation is a matter of debate and greatly relies on clinical researches especially those focusing on long term cytogenetic follow up of CML patients. Results of such studies will only re-emphasize on the clinical importance of identification of
Philadelphia chromosome during the treatment phase. The patients in our study, with Ph negative BCR-ABL positive CML belonged to the young age group and none was above 45 years of age. On the contrary, the frequency of Ph+ve CML cases was highest in age group 41-50 years (14/49 cases; 28.6%) followed by those in the age group 51-60 years (13/49 cases; 26.5%)(figure 1). Female patients with
Philadelphia chromosome positive CML were 20 (40.81%) and Ph+ve males were 29 (59.18%) (Table 1). Chavan et al 8 observed in the Indian population that the incidence of CML with Ph chromosome was predominant in the age group of 26-50 years (65.62%) and the frequency of Ph+ve CML was more preponderant in males (n=61, 63.548%) than females (n=35, 36.46%). However, Boronova et al12 detected Philadelphia chromosome in 27 men and 41 women (Russian population). This difference in observation may suggest that gender wise distribution of Philadelphia chromosome positive CML may vary in different population group around the world. One more explanation for this difference in observations may be the selection criteria employed in different studies are responsible for the characteristics of patients. However these Ph-ve CML cases were BCR-ABL positive as detected by Fluorescent in Situ Hybridization (FISH) assay prior to enrollment. Du et al13 in their study of 148 patients, found that 95.5% carried Ph chromosome. For rest 7 cases without Ph chromosome, 4 were identified as being BCR/ABL fusion gene positive. This observation is similar to ours and signifies the fact that molecular cytogenetic analysis must be done to directly visualize the rearrangement of BCR-ABL oncogene. We believe that advanced molecular studies provide deeper insights into the pathogenesis as well as reason for chromosome resistance in this type of CML and thus, are important for diagnosis and follow up of chronic myeloid leukemia cases. Moreover, molecular cytogenetic studies like FISH assay which directly visualize the rearrangement of the BCR-ABL gene are necessary to correctly define the Ph+ve or Ph-ve CML.
Meanwhile, conventional cytogenetic methods have their own advantages and remain the gold standard for detecting chromosomal aberrations.8-10 Taking into consideration the stage of CML of our study subjects, 81.6% cases of Ph+ve karyotype had chronic CML and all the patients in the accelerated and blastic phases showed the presence of Philadelphia chromosome(figure 2). All the 3 patients having normal karyogram or Philadelphia negative BCR-ABL positive CML belonged to chronic phase of Chronic myeloid leukemia. This was in resonance with the findings of Deininger et al14 who investigated 515 CML patients having clonal cytogenetic abnormalities in Philadelphia chromosome-negative cells, for their prognosis and most patients had chronic phase CML.
CONCLUSION
The Philadelphia positive BCR-ABL positive CML cases were 94.2% and the prevalence of Philadelphia positivity in CML was found to be comparable in different population groups of the world. Pertaining to disease stage, 81.6% cases of Ph+ve karyotype had chronic CML and all the patients in the accelerated and blastic phases showed the presence of Philadelphia chromosome. While, all the Philadelphia negative BCR-ABL positive CML belonged to chronic phase of CML. All the Ph negative CML cases were seen in young patients below 45 years of age. The frequency of Philadelphia positive CML cases was highest in age group 41-50 years, followed by those in 51-60 years age group and more prevalent in men with 59.18% male patients and 40.81% female patients which were different from previous studies done on other populations and/or having different selection criteria. Thus, Cytogenetic analysis done in cases of CML serve to confirm the diagnosis, sequential karyotyping helps to monitor the treatment efficacy and additional chromosomal abnormalities as and when detected, add important prognostic information. Thus, conventional cytogenetic analysis serves as a cost effective technique which can be done in every case of case of CML, and only those who are found Philadelphia negative should be subjected to molecular cytogenetic analysis to directly visualize the rearrangement of BCR-ABL oncogene.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals, and books from where the literature for this article has been reviewed and discussed.
Conflict of interest: None
Funding: None
References:
1. Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J, editors. Harrison’s principles of internal medicine. 18th ed. New York: Mcgraw hill; 2012.
2. Cavalli F, Kaye SB, Hansen HH, Armitage JO, Piccart-Gebhart MJ, editors. Textbook of medical oncology. 4th edition. London: Informa Healthcare; 2009.
3. Perrotti D, Jamieson C, Goldman J, et al. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254–2264.
4. Vardiman JW, Melo JV, Baccarani M, Thiele J. Myeloproliferative Neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edition. Lyon: IARC Press. 2008:32–37.
5. Pitchford CW, Hettinga AC, Reichard KK. Fluorescence in situ hybridization testing for −5/5q, −7/7q, +8, and del(20q) in primary myelodysplastic syndrome correlates with conventional cytogenetics in the setting of an adequate study. Am J Clin Pathol. 2010;133(2):260–4.
6. Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–50.
7. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26): 6760-68.
8. Chavan D, Ahmad F, Iyer P, et al. Cytogenetic Investigation in Chronic Myeloid Leukemia: Study from an Indian Population. Asian Pacific J Cancer Prev. 2006;(7):423-426.
9. Anand MS, Verma N, Varma S, et al. Cytogenetic and molecular analysis in adult chronic myelogenous leukemia patients in North India. Indian J Med Res. 2012;135(1):42-48.
10. Shaffer LG, McGowan-Jordan J, Schmid M, editors. ISCN (2013): An International System for Human Cytogenetic Nomenclature. Basel: S. Karger, 2013.
11. Wan TSK. Cancer cytogenetics: methodology revisited. Ann Lab Med. 2014;34:413-425.
12. Boronova I, Bernasovsky I, Bernasovska J, et al. Detection of Philadelphia chromosome in patients with chronic myeloid leukemia from the Presov region in Slovakia(1995-2004). Bratisl Lek Listy. 2007;108(10-11):433-436.
13. Du QF, Liu XL, Song LL, et al. Clinical significance of cytogenetic analysis in chronic myeloid leukemia (with report of 155 cases). Di Yi Jun Yi Da Xue Xue Bao. 2002;22(10):905-7.
14. Deininger MW, Cortes J, Paquette R, et al. The prognosis for patients with chronic myeloid leukemia who have clonal cytogenetic abnormalities in philadelphia chromosome-negative cells. Cancer. 2007;110(7):1509-19.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License