IJCRR - 6(12), June, 2014
Pages: 01-11
Date of Publication: 23-Jun-2014
Print Article
Download XML Download PDF
INHIBITION OF CORROSION OF MILD STEEL IN 1M SULPHURIC ACID BY A NEW SCHIFF BASE
Author: P.M. Dasami, K. Parameswari, S. Chitra
Category: General Sciences
Abstract:Aim: Corrosion is a serious problem faced by all the industries. The use of inhibitors is a simple and cost effective method to control corrosion. This paper reports the synthesis of a novel Schiff base and its application as corrosion inhibitor for mild steel. Methodology: Inhibition behaviour of synthesized Schiff base for the corrosion of mild steel in 1M H2SO4was studied by weight loss measurements, potentiodynamic polarization, and andelectrochemical impedance spectra. Results: The results revealed that the efficiency depends on concentration of the inhibitor and temperature. The inhibitor obeys Langmuir isotherm indicating monolayer adsorption on the mild steel surface. Thermodynamic parameters show that the adsorption of the inhibitor on mild steel surface occurs through electrostatic attraction. Polarization studies show that the inhibitor behaves as mixed type in 1M H2SO4 affecting both anodic metal dissolution and cathodic hydrogen evolution. The formation of surface adsorptive film of the Schiff base on the mild steel surface was confirmed by SEM and EDX studies. Conclusion: The prepared Schiff base is found to be a very good inhibitor for the mild steel corrosion in sulphuric acid media even at low concentration offers more than 90% inhibition efficiency.
Keywords: Inhibitors, Schiff base, Thiadiazoles, Potentiodynamic polarization, Langmuir isotherm, Impedance.
Full Text:
INTRODUCTION
Corrosion is an electrochemical process by which metallic surfaces react with their environment causing the metal to lose its material properties due to surface deterioration. The use of inhibitors is one of the most practical methods for protection of metal against corrosion, especially in acidic media.Organic compounds contain mainly oxygen, sulfur, nitrogen atoms, and multiple bonds in the molecules that facilitate the adsorption of the molecules on the metal surface1,2. The polar unit is regarded as the reaction center for the adsorption process. Thus, polar organic compounds are adsorbed on the metal surface, forming a charge transfer complex bond between their polar atoms and the metal. The size, orientation, shape and charge on the molecule determine the degree of adsorption and hence the effectiveness of the inhibitor. Various Schiff bases have been reported as effective corrosion inhibitors for steel. In the present work, the efficiency of a new synthesized Schiff base (TDIQ) as inhibitor for the corrosion of mild steel in 1M sulphuric acid was evaluated on the basis of weight loss,lectrochemical impedance spectroscopy (EIS), Tafel polarization data and SEM- EDX studies.
MATERIALS AND METHODS
Chemical composition of the mild steel The experiments were performed with cold rolled mild steel specimen of chemical composition (C=0.20%, Mn=1%,Si-0.05%,S=0.025%,P=0.25% and Fe=98%) Structure of the inhibitor (TDIQ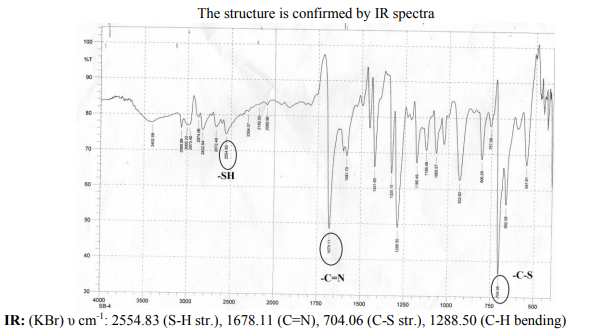
Corrosion monitoring methods Weight loss measurements were carried out with rectangular mild steel plates of dimension 3cm× 1cm× 0.1cm. The polished, preweighed plates were immersed in 1M H2SO4 containing various concentrations of the inhibitor for 3 hrs, then reweighed. From the loss in mass, inhibition efficiency was calculated. The effect of temperature on the inhibition efficiency was determined by carrying out the weight loss measurements at higher temperatures (303K-333K) The electrochemical studies were carried out with IVIUM Compactstat(potentiostat/galvanostat), using mild steel rod embedded in Teflon with exposed area 0.785 cm2 . The potential range is - 200 to +200 mV with respect to the open circuit potential at a scan rate of 1mV/sec. For impedance measurements, the sweep frequency is from 10Hz to 0.01KHz with a signal amplitude of 10mV SEM micrographs for the mild steel were recorded after 3 hrs of immersion in 1MH2SO4 solution with and without inhibitor. The synergistic effect was studied with 1mM KCl, KBr and KI in 1MH2SO4 with various concentration of the inhibitor, where inthepreweighed steel specimenwere immersed for 3 hours. From the weight loss, inhibition efficiency was calculated.
RESULTS \
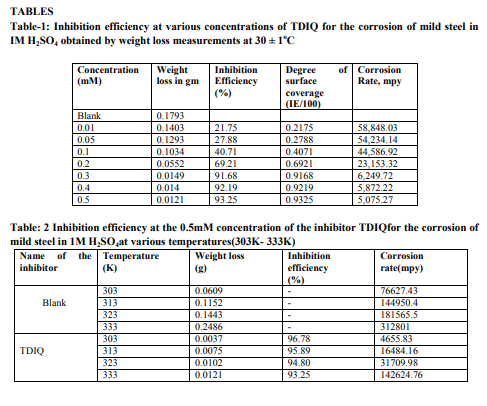
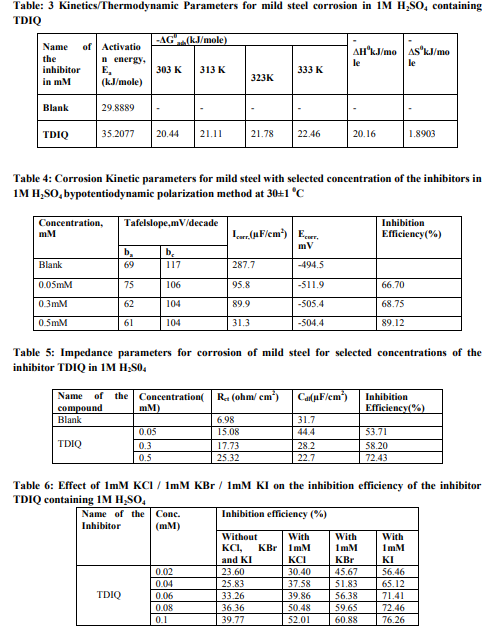
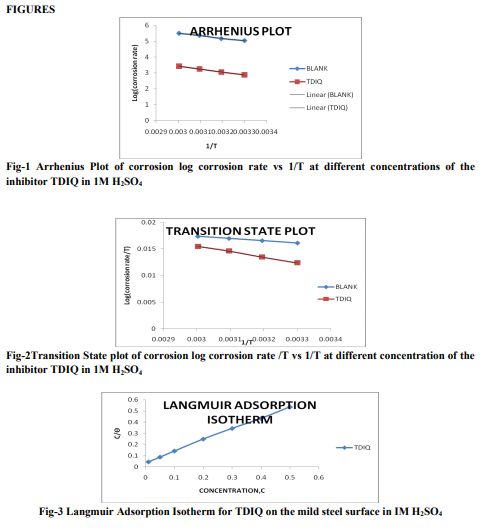
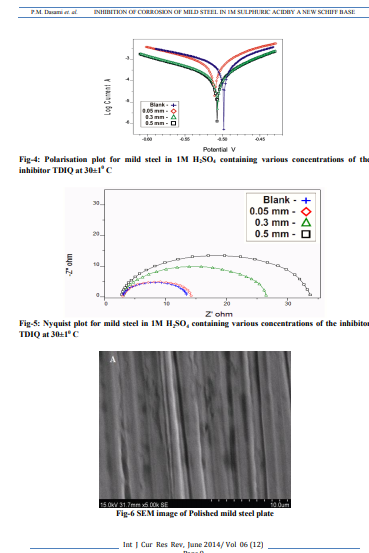
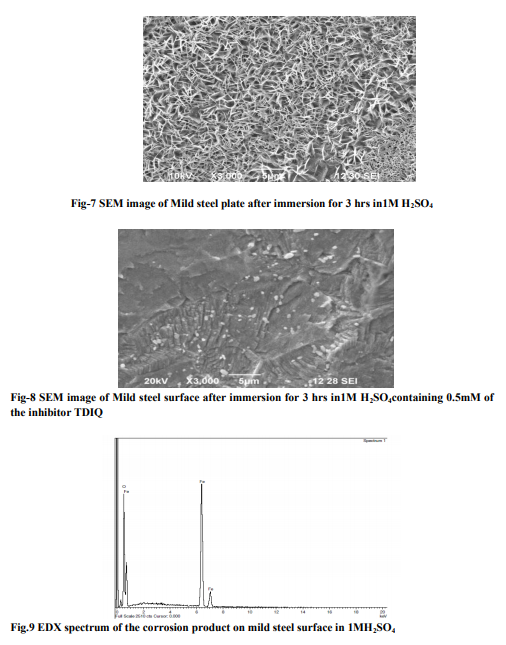
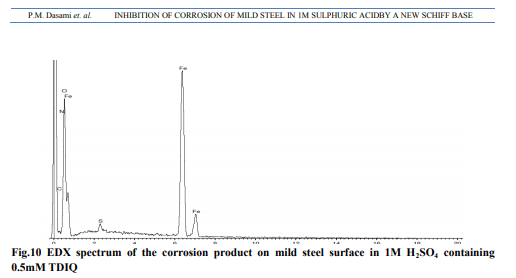
Weight loss measurements Table-1 gives the Inhibition efficiencies obtained from the weight loss measurements of mild steel for different concentrations of the inhibitor. It is clear that the inhibition efficiency increased as the concentration of the inhibitor increased from 0.1mM to 0.5mM. Effect of temperature and thermodynamic parameters In order to study the effect of temperature on the inhibition efficiency and to determine the activation energy for the corrosion process, the weight loss studies were carried out at higher temperature(303K-333K).The results are given in the Table -2. It may be noted that the inhibition efficiency decreased with temperature. The corrosion rate (CR) of the mild steel in acidic media is related to the temperature by the ArrheniusequationLog CR = log A – Ea/ 2.303 RT Where CR is the corrosion rate, Ea is the apparent activation energy, R is the molar gas constant, T is theabsolute temperature and A is the frequency factor. The plot of log CR and 1/T gives linear plots(Fig.1) and the value of Ea is obtained from the slope of the straight line (Table -3) were calculated from the slope of the straight lines. An alternative form of Arrhenius equation is Corrosion rate= RT/Nhexp(?S 0 ads/R) exp(- ?H0 ads/RT) Where h is Planck’s constant, N is Avagrado number, ?S0 is the entropy of activation and ?H0 is the enthalpy of activation. Fig.2 shows a plot of log corrosion rate against 1/T. A straight line is obtained with slope of ?H0 ads/R and interceptlnR/Nh + ?S0 ads/R from which the values of ?H0 ads, ?G0 adsand ?S0 ads are calculated and are listed inTable -3. Langmuir Adsorption Isotherm Langmuir isotherm is an ideal isotherm for physical or chemical adsorption where there is no interaction between the adsorbate and adsorbent3 . A graph plotted between concentrationvs C/? gives a linear behaviour. Assumption of Langmuir relates the concentration of the adsorbate in the bulkof the electrolyte (C) to the degree of surface coverage (?) according to the equation, C/?= 1/K + C Where ‘K’ is the equilibrium constant of adsorption. Potentiodynamic polarisation study Potentiodynamicpolarisation studies on the mild steel have been made for the inhibitor in 1M H2SO4. The electrochemical parameters such as Icorr(corrosion current) and Ecorr(corrosion potential) obtained from Tafel plot for the inhibitor (Fig. 4) is given in Table 4. The Icorrvalue decreases as compared to the blank value and at 0.5mM of inhibitor, the Ecorr value is slightly shifted towards negative direction. Tafel constants ba and bcare affected with increase in concentration of the inhibitor. Electrochemical impedance spectroscopy Nyquist plot of mild steel in uninhibited and inhibited acidic solutions containing various concentrations of TDIQ are given in Fig-5.The impedance parameters derived from Nyquist plots are given in Table -5. It was observed that there is a gradual decrease in Cdl(double layer capacitance) value with increase in concentration from 0.05 to 0.5 mM, whereas the Rct(charge transfer resistance) increases with the concentration of the inhibitor. Effect of halide ions The synergistic effect provided by the addition of halide ions such as I-, Br-, and Cl- to the solutioncontaining 1M H2SO4 and the inhibitor was studied by weight loss method and the data is presented in Table-6. Analysis of the data reveals that the addition of halides to the inhibitorincreases the inhibition at each concentration of the inhibitor used. It has been reported that the surface of iron is found to be positively charged in an inhibitor free sulphuric acid media4 . When the inhibitor is added, the nitrogen atoms present get protonated to form a cation. Hence they would be less strongly adsorbed. However, when halide ions are added,they are strongly chemisorbed on the positively charged steel surface. This makes the organic cations to be adsorbed on the layer of the anions. This is called co-operative adsorption and is responsible for the increased IE of the inhibitor in presence of halide ions. Scanning Electron Microscopy Studies The surface morphology of carbon steel surface was evaluated by Scanning Electron Microscopy (SEM). Fig. 6,7and 8show the SEM image of the polished mild steel surface, steel surface immersed in 1M H2SO4and with TDIQ.It is clear that in the absence of the inhibitor, the surface is highly corroded. However in the presence of inhibitor the rate of corrosion is suppressed. EDX Studies Energy-dispersive X-ray (EDX) spectroscopy was used to determine the elements present on the metal surface before and afterexposure to the inhibitor solution. Fig. 9 and 10 show the EDX graph for the mild steel immersed in 1M H2SO4and with TDIQ. The EDX graph shows that the presence of nitrogen, sulphur, oxygen atoms present in the inhibitor, which is adsorbed on the mild steel surface.
DISCUSSION
The inhibition efficiency increased as the concentration of the inhibitor increased.The behavior may be attributed to an increase in surface coverage,? by the adsorption of inhibitor on the mild steel surface, in the aggressive solution, which restricts the dissolution of the metal. The inhibition efficiency decreased with temperature. This may be attributed to desorption of the inhibitor molecule from metal surface at higher temperature5 .This is also supported byEa (Activation energy) value, which is higher in the presence of inhibitor as compared with that in the absence which is characteristics of physisorption of the inhibitor andformation of an absorptive film of electrostatic nature6 . Negative ?G0 ads(change in Gibb’s Free energy of adsorption) values indicate spontaneity of the adsorption process and the values are less than 40kJ/mol showing that the inhibitor is adsorbed on mild steel by physisorption. The negative sign of enthalpy ?H0 ads(change in enthalpy of adsorption) reflects the exothermic nature ofsteel dissolution. Negative entropy imply that the formation of activated complex is the rate determining step and represents an association rather than dissociation and that a decrease in disorder occurs7 . Applicability of Langmuir isotherm to the adsorption of inhibitor on mild steel confirms the formation of monolayer adsorption where there is interaction between the adsorbate and the adsorbent8 . In the electrochemical studies, Icorr is reduced in presence of Schiff base showing the efficiency of the inhibitor.Tafel constants ba and bcare affected with increase in concentration of the inhibitor suggests that the inhibitor is mixed type9 . In the EIS study, the Nyquist plots are semicircular in appearance indicating that charge transfer process controls the corrosion of mild steel10. The decrease in Cdl value results from increase in surface coverage by the inhibitor, which lead to an increase in Inhibition Efficiency (IE). The synergistic influence of halide ions is more for I- ion which may be attributed to the large size and ease of polarisibility of I- ion which facilitates stronger chemisorption with iron surface. The SEM image observations suggest that the inhibitor forms a protective layer on the surface that prevents the attack of acid on the metal11. The EDX studies inferred that the molecule interactedstrongly with the metal through sulphur atom12 . Corrosion inhibition mechanism From the obtained results of various experimental techniques used, it was concluded that the synthesized Schiff base inhibit the corrosion of mild steel in 1M H2SO4 by adsorption at the metal–electrolyte interface. Two modes of adsorption can be considered. The physical adsorption requires the presence of charged metal surface and charged inhibitor species in the solution, while chemisorption involves charge sharing or charge transfer from the inhibitor to the metal surface. TDIQ,whichposseses N and S atoms with lone pair of electrons and also π electrons of the aromatic rings. The thermodynamic parameters suggest a physisorption mechanism prevails with the studied Schiff base. The effective performance of the inhibitor may be due to the electrostatic interaction between the positively charged Fe surface and the π electron of aromatic rings and azomethine group and also the lone pair of the electron on N and S atoms.
CONCLUSION
The synthesized Schiff base is a very good inhibitor for the corrosion of mild steel in 1M H2SO4. The inhibition efficiency increases with increase in concentration of the inhibitor and decrease with temperature. The inhibitor acts by adsorption on the mild steel surface and the adsorption obeys Langmuir isotherm. Electrochemical studies show that the inhibitor is mixed type but slightly cathodic. SEM and EDX studies revealed the formation of a protective film of the inhibitor on the steel surface.
ACKNOWLEDGEMENT
Authors acknowledge theimmense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
REFERENCES
1. Bentiss F, Traisnel M, Hildebrand HF, Lagrenee M, 2,5 –bis (4- dimethylaminophenyl)-1,3,4- oxadiazoleand 2,5-bis (4-dimethylaminophenyl)1,3,4- thiadiazole as corrosion inhibitors for mild steel in acidic media.Corro. Sci. 2004; 46: 2781-2792
2. Ozcan M, Dehri I, Erbil M, Organic sulphur containing compounds as corrosion inhibitors for mild steel in acidic media: correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci., 2004; 236: 155- 164.
3. Elawady GY, El-Said IA, Fouda AS, Anion surfactants as corrosion inhibitors for aluminium dissolution in HClsolutions.Int.J.Electrochem. Sci, 2008; 3: 177-190.
4. Martinex. S, Stern. I, Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/suphuric acid system. Appl. Surf. Sci. 2002; 199: 83-89
5. Ananthkumar Ra S, Sankar A, Ramesh kumarS,Corrosion Inhibition Of Mild Steel In 0.5M H2SO4 By 1-(2-Methyl-4-(2- Methylphenyldiazenyl) Phenyl) Azonapthalen-2-ol. Am. J. of Engg Res. (AJER), 2013; Volume-02, Issue-09: 17-22.
6. PopovaS,SokolovaA, Raicheva S, ChristovM., AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazolederivatives.Corro. Sci.2003; 45: 33-58 Bockris JOM., ReddyAKN., Mod.Electrochem.,vol 2, Plenum Press, Newyork (1977) 126
7.
8. Chitra. S, ParameswariK., Vidhya. M,Kalishwari M, and SelvarajA., Sulpha Schiff bases are corrosion inhibitors for mild steel in 1M H2SO4, Int. J. Electrochem. Sci., 2011; 6: 4593
9. Bentiss. F, Lebrini M, LagreneM. , Thermodynamic characterisation of metal dissolution and inhibitor adsorption processes in mild steel /2,5-bis(n-thionyl)- 1,3,4- thiadiazoles / hydrochloric acid system,Corros. Sci2005;47: 2915-2931.
10. Jayaprabha C, Sathiyanarayanan S, VenkatachariG., Influence of metal cations in the inhibitive effect of polyaniline for iron in 0.5M H2SO4, Mater.chem.and phy 2005; 107: 350.
11. Shaju KS,Joby Thomas K, Vinod P. Raphael, and Aby Paul, Electrochemical and Surface Morphological Studiesof Carbon Steel Corrosion by a Novel Polynuclear SchiffBase in HCl Solution. ISRN Electrochem, 2013; http://dx.doi.org/10.1155/2013/820548
12. Rajappa SK, Venkatesha TV, Praveen BM, Chemical treatment of zinc surface and its corrosion inhibition studies. Bull. Mater. Sci., 2008;31: pp. 37–41.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License