IJCRR - 6(13), July, 2014
Pages: 01-05
Date of Publication: 12-Jul-2014
Print Article
Download XML Download PDF
STUDY OF ANTIMICROBIAL ACTIVITY OF NANO SILVER (NS) IN TISSUE CULTURE MEDIA
Author: Ibrahim Bala Salisu, Aminu Shehu Abubakar, Madhu Sharma, Ramesh N. Pudake
Category: General Sciences
Abstract:Plant tissue culture is a basic and fundamental component of plant biotechnology and progress in various fields of biotechnology greatly depends on the improvement of this technique. Nowadays, nanomaterials especially Nano silver (NS) are frequently being used as an antimicrobial agent in different fields of sciences including in vitro propagation of plants. Microbial contamination is one of the most serious problems in plant tissue culture and various techniques are being employed to reduce it. This study was carried out to assay the anti-microbial activities of green synthesized silver nanoparticles in the tissue culture growth mediaIn order to assay the efficacy of NS in controlling microbial contamination in the tissue culture; we first synthesized the AgNPs from the leaf extract of Lemon grass (Cymbopogon citratus). The synthesized AgNPswere characterized by UV-vis spectroscopy (UV-vis), and transmission electron microscopy (TEM). After wards, we used various rate of silver nano particles in MS media (5, 10, 20, 25, 40 ml L-1) and the sterilized explants from C. citratuswere cultured on MS medium and evaluatedafter a week interval upto the 4th week of treatment. Adding nano silver at the rate of 40 ml L-1 to growth media was fully effective to control the bacterial infection when evaluated after four weeks of culturing. The finding of this study indicates that NS has a good potential in eliminating of the bacterial contaminants in plant tissue culture.
Keywords: Antimicrobial activity, Nano Silver, Biosynthesis, Cymbopogon citratus, Tissue Culture
Full Text:
INTRODUCTION
Nanotechnology is one of the most power full areas of research in contemporary materials science. Nanoparticles entirely display novel or better properties on basis of some important unique features such as size, distribution and morphology. Recent applications of nanoparticles and nanomaterials are emerging rapidly. Nano crystalline silver particles have found large applications in high sensitivity bio molecular detection, diagnostics, therapeutics, microelectronics, catalysis, and also as antimicrobials (Jain et al. 2009). The use of Nano silver is rapidly increasing in consumer products due to its synthesis simplicity and can be easily produced on a large scale.The potential of silver as a medicine or traditional anti-microbial agent particularly bacteria was not realized until nineteenth century. Since then, the antimicrobial activity of silver has been studied and more extensively used than any other inorganic antibacterial agent. Silver nanoparticles (NPs) are found to be toxic to bacteria, and are presently used in various devices likemedical devices, washing machines etc. to inhibit microbial growth (Li et al. 2008) The toxicity of Silver is due to its ability to attack a wide range of biological processes in microorganisms leading to the disruption cell membrane structure and collapse of plasma membrane potential which depletes the intra cellular ATP thereby ensuring the cell death. Small amount in micro molar (1 to 10 μM) of silver ions can efficiently inhibit bacterial growth in water. However at higher doses silver can be harmful to mammals, freshwater and marine organisms, and such micro molar amount of silver is non-toxic humans. Hence, silver has been used extensively for the development of many biological and pharmaceutical processes, products, and appliances such as coating materials for medical devices, orthopedic or dental graft materials, and topical aids for wound treatment, water sanitization, textile products, and even washing machines (Jo et. al. 2009). The use of silver nano particles as antimicrobial agents has become more common due to the technological advancement, whichresulted into an economical production. The potential of silver as antimicrobial agent is extensively used in plant diseases control as well as the management of various plant pathogens in a relatively safer way as compared to synthetic fungicides (Jo et. al. 2009). Currently different strategies such as the use of antibiotics have been developed to eliminate microbial contamination during in vitro propagation. However, studies have shown that antibiotics are frequently toxic to plants and may otherwise delay or even inhibit the growth of plant tissues (Abdi et. al. 2008). For example, Streptomycin and chloramphenicol are inhibitors of protein synthesis; while nucleic acid synthesis is inhibited by quinolone and rifampicin, and penicillin inhibits cell-wall membrane synthesis (Kohanski et. al. 2010). Due to the continuous used of antibiotics; bacteria are developing resistance and becoming insensitive. The use of silver nanoparticlescan be considered as good alternative (Rai et. al. 2012), as at low concentration it possesses antimicrobial activity (Safavi et. al. 2011). The use of green synthesized nanoparticle in various fields is receiving attention from many researchers because of their ecofriendly nature Deepak et al. 2011). The present study was conducted to assay the anti-microbial activities of green synthesized silver nanoparticles in plant growth media.Lemon grass was selected for its richness in plant phytochemicals such as flavonoids, alkaloids, saponins, alcohol, ketones, aldehydes, steroids, terpenes, phenols (Asaolu et. al. 2009; Shah et. al. 2011; Sofowora et al.1982) which serve as reducing agent in reducing Ag+ to nanosilver.
MATERIAL AND METHODS
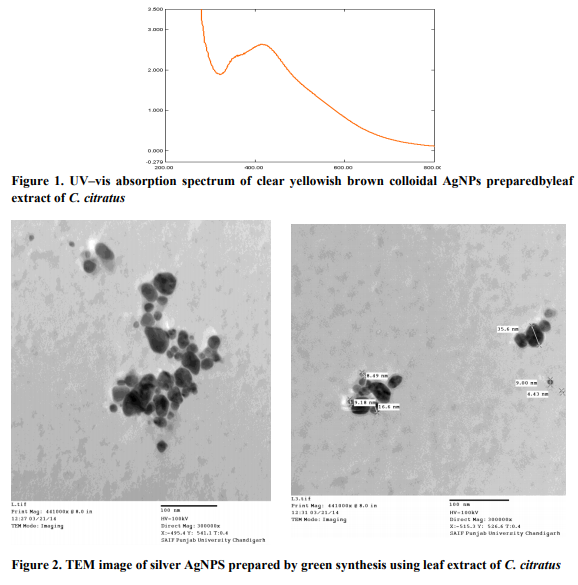
Preparation of plant extract Fresh leaves of Cymbopogon citratus were collected from the field and washed thoroughly with sterile distilled waterbeforechopped into small pieces. Afterwards 10 g of clean chopped leaves were taken into a flask with 100ml sterile double distilled water and boiled for 5 minute. The extract was decanted and then filtered using Whatman filter paper and used as reducing agent during the green synthesis of AgNP. Preparation of silver nanoparticles 1mM aqueous solution of silver nitrate (AgNO3) was prepared and used for the synthesis of silver nanoparticles. 10 ml of Cymbopogon citratus extract was taken and added into 90 ml of aqueous solution of 1 mM Silver nitrate and incubated overnight at room temperature in the dark. Brownish yellow solution was formed, indicating the successful formation of silver nanoparticles. UV-visible spectrum analysis Equal amount of sample aliquot and distilled water(1ml each) were mixed in a 10 mm-opticalpath-length quartz cuvettes, and the UV-vis spectrum analysis of the reaction mixture was carried out to detect the reduction of pure Ag+ ions. The concentration of AgNPs produced was measured using a Systronics UV double beam spectrophotometer, at a resolution of 1 nm, between 200 and 800 nm (Figure 1).
Transmission electron microscopy (TEM) analysis
TEM analysis was used to get the micrograph image of the green synthesized AgNPs (Figure 2). Modification of plant tissue culture media by nanomaterial Different amounts of nano silver were added to tissue culture media. Five different levels of NS (5, 10, 20, 25 and 40 ml/L) were added into the Murashige and Skoog (MS) medium (Murashige and Skoog, 1962). The nanoparticles were added after autoclaving and afterwards thesterilized explants (nodal segment) from C. citrates were inoculatedonto the medium. A completely randomized design was used with each treatment was replicated six times. The cultures were monitored, observations were made and records were takenup to four weeks (1, 2, 3 and 4) after inoculation.The percentages of inoculated media that showed a growth or sign of microbial growth were estimated.
RESULTS AND DISCUSSION
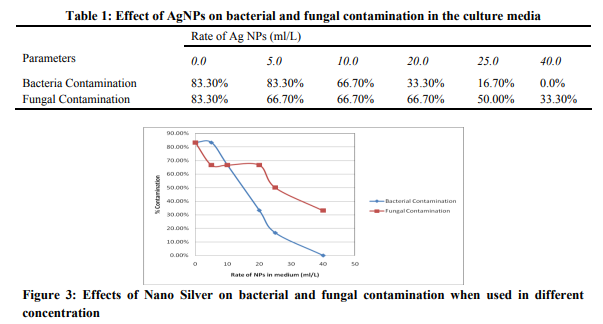
The detailed study on extracellular biosynthesis of AgNP using the leaf extract of C. citratus extract was carried out with the anti- bacterial effects in tissue culture medium. Results of this study (Table 1) showed that addition of Nano-Silver to tissue culture media significantly reduce bacterial contamination compared with the control. The use of the 40mlL-1 1mM concentration of the AgNps in the MS medium eliminated bacterial contamination (0.0%). Increasing the amount of the nanoparticles in the media from 10ml to 20 ml significantly decreased the rate of bacterial contamination from 66.70 % to 33.30% and subsequently to 16.30% when 25ml was added. Total elimination of fungal contamination was however not achieved even at the highest rate of the AgNPs used (40 ml/L), but use of AgNPs showed a decreased fungal growth ascompared to control. 40ml/L significantly reduced the rate of fungal contamination from (83.3% to 33.3%). Similar finding was also reportedin previous studies where a 100mg/Lof AgNPs in plant tissue culture controlled bacterial growth (Safavi et. al. 2011). It is evident from this study that, higher concentration AgNPs is required to effectively controlling fungal growth. This is also in line with what had been reported in previous studies, likeaconcentration of 200 mg L-1 AgNPs successfully controlled bacterial and fungal contamination without any harmful effects on regeneration of the lemon grass explants (Fakhrfeshani et. al. 2012). It has also been reported that NS can be an efficient tool for removing contaminants from plant tissues, only if the right dose and exposure time are to be used (Mahna et. al. 2013). Nevertheless, NS has not yet become a universal decontamination agent, and our finding will help in future research in finding the effective way of controlling microbial contamination in plant tissue culture.
CONCLUSION
The use of NS in tissue culture media as a substitute of antibiotics to control microbial contaminations is becoming an interesting area of study. In this research the antimicrobial activity of nano silver was studied, and our results confirm its ability to reduce the microbial growth in the MS media and can allowed the explants to grow successfully when appropriate concentration is used. These results will be helpful in the refinement of protocol for use of NS particles in in vitro multiplication of various plants.
ACKNOWLEDGMENTS
The authors would like to thanks Mrs. Geetika Sahni, Assistant Professor, Lovely professional University, Phagwara for providing the necessary support for successful completionof this experiment. Authors also acknowledged the authors, journals and publishers whose articles were cited in this work.
References:
1. Abdi G, Salehi H, Khosh-Khui M. Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol Plant 2008; 30: 709-714.
2. Asaolu MF, Oyeyemi OA, Olanlokun JO. Chemical compositions,phytochemical constituents and in vitro biological activity of various extracts ofCymbopogon citratus, P.J. Ntr, 2009; 8(12): 1920-1922.
3. Deepak V, Kalishwaralal K, Pandian SRK, Gurunathan S. An insight into the bacterial biogenesis of silver nanoparticles, industrial production and scale-up In Rai, M., Duran, N. (Eds), Metal nanoparticles in microbiology 2011; pp 17-35. http://www.springer.com/978- 3-642-18311-9
4. Fakhrfeshani M, Bagheri A, Sharifi A. Disinfecting effects of nano-silver fluids in Gerbera (Gerbera jamesonii) capitulum tissue culture. J. Biol. Environ Sci2012; 6: 121-127. http://dx.doi.org/10.4172/2157-7439.1000161
5. Jain D, Kumar H, Daima S, Kachhwaha S, Kothari L. Synthesis of Plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. A. Digest Journal of Nanomaterials and Biostructures 2009; 4: 557 – 563.
6. Jo YK, Kim BH, Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Disease 2009; 93:1037-1043.
7. Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 2010; 8(6): 423- 435.
8. Li Q,Mahendra SH, Lyon DY, Brunet L, Liga MV, Li D. Alvarez PJJ. Antimicrobial Nanomaterial for water disinfection and microbial control: Potential applications and Implications. J Inter water research 2008; 42.
9. Mahna N, Vahed SZ, KhaniS. (2013). Plant in vitro culture goes nano: Nano silver-mediated decontamination of ex vitro explants: J Nanomed Nanotech., 4:2.http://dx.doi.org/10.4172/2157- 7439.1000161.
10. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco Tissue cultures. Physiol Plant 1962; 5:473-97.
11. Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol 2012; 112(5): 841- 52.
12. Safavi K, Esfahanizadeh M, Mortazaeinezahad DH, Dastjerd H. The study of Nano-Silver (NS) antimicrobial activity and evaluation of using NS in tissue culture media. Inter Conference on Life Sci and Tech, IPBCEE 2011.
13. Shah G, Shri R, Panchal V, Sharma N, Singh B. Scientific basis for the therapeutic useof Cymbopogon citratus. J Adv Pharm Technol Res 2011; 2(1), 3–8.
14. SofoworaEA, OlaniyiAA, Oguntimehin BO. Pyhtochemical Investigation of some Nigerian Plants used against fevers. Planta Med 1982; 28: 186-189.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License