IJCRR - 6(20), October, 2014
Pages: 48-54
Date of Publication: 20-Oct-2014
Print Article
Download XML Download PDF
POSTOPERATIVE NAUSEA AND VOMITING: A REVIEW
Author: Dhruva Sharma, Neha Sharma, Ajitesh Kumar Mishra, Preksha Sharma, Neelima Sharma, Pooja Sharma
Category: Healthcare
Abstract:Postoperative Nausea and Vomiting (PONV) was termed \"the big little problem\" nearly a quarter century ago in an editorial (Kapur et al, 1991). The past decade has witnessed the introduction of several significant innovations to combat PONV, particularly the introduction of serotonin antagonists and the use of combinations of drugs for analgesia and control of PONV. But it still remains as big a problem as before because newer choices and confusions over standardization added side by side. PONV remains a significant problem in modern anesthetic practice also because of adverse consequences such as delayed recovery, unexpected hospital admission, delaqayed return to work of ambulatory patients, pulmonary aspiration, wound dehiscence, and dehydration PONV is controlled by the emetic, or vomiting centre, in the brain. Stimuli are also sent from the cerebral cortex and chemoreceptor trigger zone (CTZ), which is situated in the brainstem. PONV is generally influenced by multiple factors that are related to patient, surgery and anesthesia and which requires release of 5-hydroxytryptamine (5- HT) in a cascade of neuronal events involving both the central nervous system and the gastrointestinal tract. The 5-HT subtype 3 receptor (5-HT3) participates selectively in the emetic response. Patients might become extremely distressed, which in turn can cause them anxiety about undergoing further surgery. PONV also has cost implications in terms of nursing time, delayed recovery, hospital resources and possible re-operation costs. In the present scenario, though we have better understanding and knowledge about the pathophysiology of nausea and vomiting and use of more stable and effective anti-emetics, the postoperative nausea and vomiting (PONV) continues to be the most disturbing complication following surgery and anesthesia.
Keywords: Postoperative Nausea and Vomiting (PONV), 5-Hydroxytryptamine
Full Text:
INTRODUCTION
POSTOPERATIVE NAUSEA AND VOMITING
Postoperative Nausea and Vomiting (PONV) was termed “the big little problem” nearly a quarter century ago in an editorial (Kapur et al, 1991) [1]. The past decade has witnessed the introduction of several significant innovations to combat PONV, particularly the introduction of serotonin antagonists and the use of combinations of drugs for analgesia and control of PONV.[2] But it still remains as big a problem as before because newer choices and confusions over standardization added side by side.[2] PONV remains a significant problem in modern anesthetic practice also because of adverse consequences such as delayed recovery, unexpected hospital admission, delayed return to work of ambulatory patients, pulmonary aspiration, wound dehiscence, and dehydration .[3]
NAUSEA, VOMITING, AND RETCHING
Nausea, vomiting, and retching are distinct concepts. However, terms to describe them often are used interchangeably, which may result in imprecise assessment, measurement, and education. [4] Nausea By definition nausea is “the feeling of a need to vomit”.[5] Nausea is a non-observable phenomenon of an unpleasant sensation experienced in the back of the throat and the epigastrium that may or may not culminate in vomiting; it is synonymously described as feeling “sick at stomach”.[6] In short, nausea is an unpleasant sensation that commonly precedes vomiting. It is usually determined through self-report but also may have some objective elements, depending on intensity. A visual analogue scale for nausea is also prescribed (Boogaerts et al, 2000) analogous to that widely used for pain measurement.[7] Rectching While nausea is an unpleasant sensation of the urge to vomit; retching involves spasmodic contractions of respiratory muscles without the expulsion of gastric content. Thus retching is the attempt to vomit without bringing anything up. As retching is gastric and esophageal movement of vomiting without expulsion of vomitus, it is described by such terms as “gagging,” “dry heaves,” and “attempting to vomit without results’’.[4]
Vomiting
The final act of vomiting is a reflex – actually an important defense mechanism for the expulsion of toxins. Thus vomiting is the forceful expulsion of the contents of the stomach through the oral or even nasal cavity. Thus vomiting involves the contraction of the abdominal muscles resulting in an expulsion of the stomach contents from the mouth .[8] Both the occurrence and the frequency of vomiting may be objectively measured. [9] The Rhodes index of nausea, vomiting, and retching (RINVR) is a method of quantifying nausea and vomiting objectively in patients who receive anti-cancer therapy.[10]
The neuroanatomical site which controls nausea and vomiting is basically an ill-defined region called the “vomiting center” which is situated within the lateral reticular formation in the brainstem. [18] It receives afferent inputs from higher cortical centers, the cerebellum, the vestibular apparatus, and vagal and glossopharyngeal nerves.[9]Further interactions occur with the nucleus tractus solitarius and the CTZ which is located in the floor of the fourth ventricle. The CTZ is outside the blood-brain barrier and in contact with cerebrospinal fluid (CSF). The CTZ enables substances in the blood and CSF to interact. [9][18] Not only direct stimulation of the CTZ induce PONV but immunochemical studies of these anatomical sites shows that these areas contain histamine, serotonin, cholinergic, neurokinin-1, and D2 dopamine receptors which results in vomiting. [9][18] The “vomiting reflex” is precipitated by different stimulation from the glossopharyngeal, hypoglossal, and vagal nerves reaching the vomiting center.[9][18] Efferent signals are directed to the glossopharyngeal, hypoglossal, trigeminal, accessory, and spinal segmental nerves. There is a coordinated contraction of abdominal muscle against a closed glottis, which raises intra-abdominal and intrathoracic pressures.[9][18] The pyloric sphincter contracts and the esophageal sphincter relaxes, and there is active antiperistalsis within the esophagus, which forcibly expels the gastric contents. This is associated with marked vagal and sympathetic activity leading to sweating, pallor, and bradycardia.[9][18] PONV is generally influenced by multiple factors that are related to patient, surgery and anesthesia and which requires release of 5-hydroxytryptamine (5- HT) in a cascade of neuronal events involving both the central nervous system and the gastrointestinal tract. The 5-HT subtype 3 receptor (5-HT3 ) participates selectively in the emetic response.[19] Otherwise too, seeing multiple mechanism and receptors physio-pathologically involved in PONV, a combination of antiemetics may be necessary – esp in the high risk groups and/ or refractory cases.[20]
PHARMACOLOGICAL FACTORS INFLUENCING
PONV Pre-medications are administered to provide sedation, anxiolysis, analgesia, reduces secretions and cardiovascular responses during induction. Sevoflurane, transdermal scopolamine and benzodiazipines are preferred to avoid PONV.[21][22][23] Many of the drugs used in anesthesia and pain control (esp opioids) cause PONV because chemoreceptors in the CTZ monitor substances in the blood and cerebrospinal fluid. The use of opioids for pain relief stimulates the vomiting centre via the CTZ. [14] They also decrease gut motility causing distension. Opioids can increase the sensitivity of the middle ear to movement which can cause nausea in some people. This explains their association with travel sickness.[14] Paracetamol alone is a sufficient pain killer in tonsillectomy and thereby also helps in reduction of anxiety and associated PONV .[24] Even reversal of skeletal muscle relaxants like curares may need neostigmine and being an anticholinesterase, it increases acetylcholine level sufficient to induce PONV. [26] Inhaled anesthetic agents, such as nitrous oxide, increase the risk of PONV. Nitrous oxide causes gut distension and pressure on the middle ear, which can both contribute to PONV.[14][26] Twenty four of twenty seven studies show a greater incidence of emesis associated with nitrous oxide than with alternative anesthetics.[27] Preoperative clonidine has also been preferred over midazolam as a sedative premedication as it is better effective against PONV, specially in children.[28] Glycopyrrolate intravenously before spinal anesthesia in caesarean section effectively controls the PONV.[29] Gabapentin, an anticonvulsant used in pains like tic doloreaux has also been proposed for PONV under similar justification of pain modification.[30] And this anticholinergic benefit considered with antiadrenergic benefit of clonidine supports the idea that reactionary (and homeostatically compensatory) hyperactivity of parasympathetic system which follows ‘sympathetic
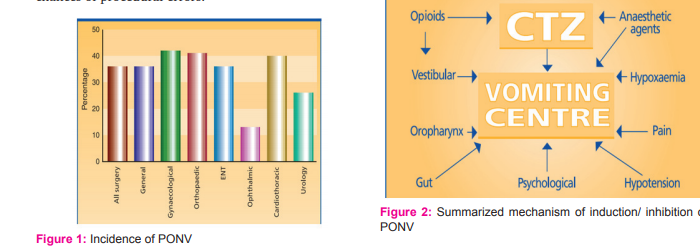
INCIDENCE OF PONV
The depicted bar diagram in figure-1 shows a general prevalence of PONV as per surgery type and in surgery overall. [11][12] Yet, among other surgeries, some procedures like tonsillectomies, strabismus surgery, laparoscopic cholecystectomies are associated with higher incidence of PONV[13], may be due to inherent increased chances of procedural errors.
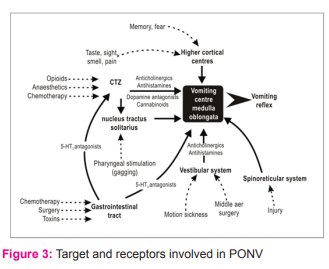
MECHANISM OF PONV
PONV is controlled by the emetic, or vomiting centre, in the brain. Stimuli are also sent from the cerebral cortex and chemoreceptor trigger zone (CTZ), which is situated in the brainstem (Jolley, 2001). The vomiting centre receives messages via the nervous system from different sources, including the pharynx, gastrointestinal tract, eye, vestibular apparatus in the ear, respiratory and circulatory systems, testicles and pain receptors. [14] Primary control of nausea and vomiting arises from the “central pattern generator for vomiting,” located in the medulla oblongata. There are five primary afferent pathways involved in stimulating vomiting (Kakuta et al, 2011): 1. the chemoreceptor triggering zone (CTZ) 2. the vagal mucosal pathway in the gastrointestinal system, 3. neuronal pathways from the vestibular system, 4. reflex afferent pathways from the cerebral cortex, and 5. midbrain afferents. Stimulation of one of these afferent pathways can activate the sensation of vomiting via cholinergic (muscarinic), dopaminergic, histaminergic, or serotonergic receptors .[15] The vomiting centre can also be stimulated by disturbance of the gut or oropharynx, movement, pain, hypoxaemia and hypotension. Because many different factors contribute to PONV, it can be difficult to prevent and treat.[14] The schematic diagram depicted in Figure-2 [16] shows the main targets of induction/ inhibition of PONV. Better explanation on the receptor level is explained in Figure-3.[17]
hyperactivity of sympathetic system during surgery’ may be a mechanism of PONV. Some of the older intravenous induction agents such as thiopentone are associated with PONV, whereas propofol has a lower incidence of PONV.[31][32] Because of the short duration of action, it is known that propofol does not show enough anti-emetic effect for PONV and in late post operative period (by now, even emetogenic effect of inhalant anesthetics might have vanished) it doesn’t differ significantly from inhalational anesthesia.[31]
NON-PHARMACOLOGICAL FACTORS INFLUENCING PONV
Differences exist in risk factors of postoperative nausea vs vomiting. The authors reported that female gender, non-smoking status, and general anesthesia increase both PONV; whereas a history of migraine and the type of surgery tend to influence nausea only .[33] And that’s why there remain many contradictory reports concerning contribution of a given factor influencing the post surgical emetic responses when patients are categorized by a carpet approach of PONV (in adults) or POV (in children).[33] 1. Age – Usually children have a higher incidence than adults but the lowest incidence occurs in infants (5%). Children aged > 3 years have an average vomiting incidence of 40%—almost twice as frequent as the rate in adults .[34] 20% of cases are seen in preschool children with a peak incidence in school going children (34-50 %).[8][35] Sex differences in risk of vomiting are not seen in children before puberty.[34]The incidence of PONV reaches a peak between 5 and 9 years of age .[36] 2. Sex – Females are more prone to PONV (Rowley et al, 1982). and women are three times more likely than men to experience PONV .[12][37] Hawthorne et al (1995) has suggested that the predictive value of female gender diminish following menopause, when the risk to each sex becomes equal. [38] The incidence of postoperative nausea and vomiting in women undergoing laparoscopy is found to be aggravated during menstruation.[39][40] Interestingly, droperidol was later shown to be ineffective in men while benefitting females irrespective of the phase of menstrual cycle – earlier studies failed to exhibit it because of sex limited (women only) use or smaller sample size .[41] Type of surgical procedure – The type of surgery performed also has an influence on the incidence of PONV and it is independent of other factors .[8] Gynecological and abdominal surgeries are more prone to PONV.[12] Later, surgery type as a factor is denied through systematic meta-analysis.[42]Rather high risk patient [42] or intraoperative hypotension has also been implicated in PONV.[34] Pneumoperitoneum induced by laparoscopy can stimulate vagal response and induce release of various emetogenic substances such as 5-hydroxytryptamine and acetylcholine and hence increase nausea and vomiting.[43] General anesthesia increases the risk of PONV 11?fold compared to regional anesthesia.[34] It is reported that the incidence of PONV is higher in laparoscopy procedures compared with laparotomy procedures.[44] Thus laparoscopic surgeries in females are most risky concerning PONV.[8] Surgical factors also include the effects of intraperitoneal CO2 insufflation on residual stretching and irritation of the peritoneum.[45] The patients undergoing ophthalmologic surgery that involves extensive manipulation of extra-ocular muscles are even more prone to develop post-operative nausea and vomiting because of the oculo-emetic reflex.[46] Excessive nausea and vomiting may interfere with post-operative care. They lead to increase in the intra-ocular pressure (IOP), which in turn may cause ocular morbidity.[46] In addition, vitreoretinal (VR) surgery often requires intra-operative administration of air, airgas or silicon oil into the vitreous cavity for prevention of post-operative tamponade.[46] These patients need to be nursed in prone position. Presence of PONV does not allow the patients to be in prone position. In such cases, ketoprofen with centrally and peripherally mediated analgesic activity can safely replace opioids as pain killer[46] In adults, many of the short-duration ophthalmologic surgeries are undertaken under regional anesthesia obviating the need of opioids as pain killer which could further aggravate PONV.[46] As regional anesthesia is not feasible in children and young adults, ketoprofen can be chosen. It has liposomal membrane stabilizing action and antibradykinin activity and inhibitory effects on leukotriene synthesis. Thus ketoprofen acts rapidly, producing analgesia within 10 minutes from an intravenous bolus dose.[46] 3. Duration of surgery- PONV increases with duration of surgery and anesthesia because of greater accumulation of emetogenic anesthetic agents .[8] [47] 5. Previous history of PONV- Greater complications has been seen in patients with previous history of motion sickness and PONV.[48]
Either due to previous experience of PONV or because of general fears about a hospital admission, apprehension can increase the likelihood of PONV occurring. This might be because of conditioning or learned responses.[14] 6. Gastric distension- Increased incidences of PONV have been seen in patients with gastric distension. The use of N2 O during laparoscopic procedures has been considered to be an important problem because of its propensity to produce bowel distension during the surgery and to increase the incidence of PONV.[49] Emergency, in which patient is operated without “empty bowel since the night before”), can also be a factor for PONV.[14] 7. Smoking status – non-smokers are more prone to PONV – it might be due to gradual desensitization of CTZ due to continued smoking which initially and universally induces nausea and vomiting .[41] 8. Other than drugs being used before, during or after anesthesia and surgery, an important confounding factors can be obesity (lipid soluble drugs may get deposited in the adipose tissue and continue longer to causes this ADR) .[14] But some studies deny the role of body mass index in PONV .[2] 9. Postoperative pain. It is very important to manage postoperative pain as it can prolong PONV by increasing gastric emptying time .[22]At the same time, prevention of PONV in surgical patients gets similar priority to that of alleviating postoperative pain .[3] Patient controlled analgesia (PCA) is a common measure against post operative pain but PCA is not without side effects and pain relief with opioids is achieved at the expense of PONV, which is a commonly reported symptom.[50]
IMPACT OF PONV
Problems associated with vomiting are loss of fluid and electrolytes, exhaustion, soreness and patient distress . [14] The negative impact of PONV on patient’s physical, metabolic and psychological condition not only delays discharge from or cause re-admission to hospital but also decreases the confidence level in future surgery and anesthesia .[5] S. Chatterjee et al 2011 documented that an episode of vomiting prolongs postanesthetic care unit (PACU) stay by about 25 minutes and even patients were willing to pay at their own expense, for a completely effective antiemetic.[18]
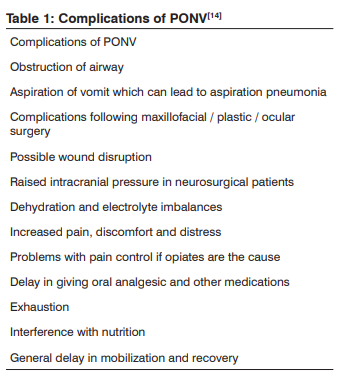
It is estimated that approximately 0.2% of all patients may experience intractable PONV leading to increased medical costs.[18] Vomiting also increases the risk of esophageal perforation, and bleeding. The increased abdominal pressure during emesis may cause tension on suture lines resulting in incisional hernia.[41] As well as medical complications, nausea and vomiting can have psychological effects on patients, such as discomfort and distress; shame and embarrassment; exhaustion; dissatisfaction with the outcome of the operation; and fear of further surgery .[14] Research has shown that nausea and vomiting are feared far more in comparison to post-operative pain, and PONV is ranked as a major concern by the most surgical patients .[41] Each episode of emesis delays discharge from the recovery room nearly by 20 minutes .[34] Patients might become extremely distressed, which in turn can cause them anxiety about undergoing further surgery. PONV also has cost implications in terms of nursing time, delayed recovery, hospital resources and possible re-operation costs .[14]
CONCLUSION
In the present scenario, though we have better understanding and knowledge about the pathophysiology of nausea and vomiting and use of more stable and effective anti-emetics, the postoperative nausea and vomiting (PONV) continues to be the most disturbing complication following surgery and anaesthesia.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Kapur PA. The big ‘little problem’. Anesth Analg 1991;73: 243 –245.
2. Kranke, Peter, and Leopold HJ Eberhart. “Possibilities and limitations in the pharmacological management of postoperative nausea and vomiting.” European Journal of Anaesthesiology (EJA) 28, no. 11 (2011): 758-765.
3. Swaika Sarbari, Anirban Pal, Surojit Chatterjee. Ondansetron, ramosetron, or palonosetron: Which is a better choice of antiemetic to prevent postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy? Anesthesia assays and researchers 2011;5( 2): 182-186.
4. Verna A. Rhodes EdS, Dr. Roxanne W. McDaniel . Nausea, Vomiting, and Retching: Complex Problems in Palliative Care.CA Cancer J Clin 2001;51(4):232–248.
5. Prunty, Leesa M. “An Outpatient Approach to Nausea and Vomiting.” US Pharm38, no. 12 (2013): 24-28.
6. Rhodes, Verna A., and Roxanne W. McDaniel. “Nausea, vomiting, and retching: complex problems in palliative care.” CA: A Cancer Journal for Clinicians 51, no. 4 (2001): 232-248.
7. Boogaerts JG, Vanacker E, Seidel L, et al: Assessment of postoperative nausea using a visual analogue scale. Acta Anaesthesiol Scand 2000; 44:470 – 474.
8. Watcha MF, White PF. Postoperative nausea and vomiting: its etiology, treatment and prevention. Anesthesiology. 1992;77:162–84.
9. Islam S, Jain PN. Post-operative nausea and vomiting (PONV): a review article. Indian J Anaesth 2004; 48 : 253- 8.
10. Kim TH, Choi BM, Chin JH, Lee MS, Kim DH, Noh GJ. The reliability and validity of the Rhodes index of nausea, vomiting and retching in postoperative nausea and vomiting. Korean J Anesthesiol 2007; 52: S59-65.
11. Ernst E (1994) The Economics of Quality Care. Postoperative Nausea and Vomiting. Cookham, Direct Publication Solutions.
12. Rowbotham D (1995) Recognising risk factors. Nursing Times. 91, 28, 44-46.
13. Mihara, T., K. Tojo, and T. Goto. “Re-evaluation of the effectiveness of ramosetron in preventing post-operative nausea and vomiting: a meta-analysis without Fujii et al.’s RCTs: ESAPC1-2.” European Journal of Anaesthesiology (EJA) 30 (2013): 1-1.
14. Jolley, Sue. “Managing post-operative nausea and vomiting.” Nursing Standard 15, no. 40 (2001): 47-52.
15. Kakuta, Nami, Yasuo M. Tsutsumi, Yousuke T. Horikawa, Hiroaki Kawano, Michiko Kinoshita, Katsuya Tanaka, and Shuzo Oshita. “Neurokinin-1 receptor antagonism, aprepitant, effectively diminishes post-operative nausea and vomiting while increasing analgesic tolerance in laparoscopic gynecological procedures.” J Med Invest 58, no. 3-4 (2011): 246-51.
16. Kenny G, Rowbotham D (Eds) (1992) Postoperative Nausea and Vomiting. London, Synergy Medical Education.
17. tkenhead AR, Rowbotham DJ, Smith G. Textbook in Anaesthesia, 5 Edition. London: Churchill Livingstone Elsevier; 2007
18. Chatterjee, S., A. Rudra, and S. Sengupta. “Current concepts in the management of postoperative nausea and vomiting.” Anesthesiology research and practice 2011 (2011).
19. Baisakhi Laha, Avijit Hazra, S Mallick. Evaluation of antiemetic effect of intravenous palonosetron versus intravenous ondansetron in laparoscopic cholecystectomy: A randomized controlled trial.Ind J Pharmacol 2013; 45(1 ): 24-29.
20. Habib, Ashraf S., and Tong J. Gan. “Evidence-based management of postoperative nausea and vomiting: a review.” Canadian Journal of Anesthesia51, no. 4 (2004): 326-341.
21. Horimoto Y, Tomie H, Hanzawa K.Scopolamine patch reduces postoperative emesis in paediatric patients following strabismus surgery. Can J Anaesth 1991;38:441-4.
22. Rose JB, Watcha MF. Postoperative nausea and vomiting in paediatric patients. Br J Anaesth 1999; 83: 104–17
23. Splinter WM, Rhine EJ, Roberts DJ. Vomiting after strabismus surgery in children: ondansetron vs propofol. Can J Anaesth 1997; 44: 825–9
24. Sen, B., S. Dogru, Nursen Koltka, and M. Gura. “The effect of intra-operative paracetamol on post operative pain, nausea and vomit in children who underwent adenotonsillectomy.” Göztepe T?p Dergisi 27, no. 1 (2012): 16-21.
25. Rother, Catriona. “Post-Operative Nausea & Vomiting-Use of Anti-Emetic Agents in Anaesthesia.” Scottish Universities Medical Journal 1, no. 1 (2012).
26. Hovorka J, Korttila K, Erkola O.The experience of the person ventilating the lung does influence postoperative nausea and vomiting.Acta Anaesthesiol Scand.1990;34:203-5.
27. Hartung J.Twenty four of twenty seven studies show a greater incidence of emesis associated with nitrous oxide than with alternative anaesthetics.Anaesth Analg 1996;83:114-16.
28. Javaherfroosh, F., M. Raza Pipelzadeh, and M. Namazi. “Clonidine reduces post operative nausea and vomiting in laparoscopic gynecological surgery.” Pak J Med Sci 25, no. Part I (2009): 782-5.
29. Biswas, B. N., A. Rudra, S. K. Das, S. Nath, and S. C. Biswas. “A comparative study of glycopyrrolate, dexamethasone and metoclopramide in control of post-operative nausea and vomiting after spinal anaesthesia for caesarean delivery.” Indian J Anaesth 47 (2003): 198-200.
30. Soroush, Ahmadreza, Hosein Masoomi, Zhamak Khorgami, Seyed MojtabaMarashi, and Roza Mofid. “Effect of prophylactic gabapentin on post operative nausea and vomiting after laparoscopic cholecystectomy: a randomized controlled trial.” Journal of Minimally Invasive Surgical Sciences 2012, no. 1, Winter (2012): 17-20.
31. Shinn, Helen Ki, Mi Hyeon Lee, Sin Yeong Moon, Sung-Il Hwang, Choon Soo Lee, Hyun Kyoung Lim, and Jang-Ho Song. “Post-operative nausea and vomiting after gynecologic laparoscopic surgery: comparison between propofol and sevoflurane.” Korean journal of anesthesiology 60, no. 1 (2011): 36-40.
32. Tate S, Cook H (1996) Postoperative nausea and vomiting 1: physiology and aetiology. British Journal of Theatre Nursing. 5, 16, 962-966.
33. Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology 2003; 98: 46–52
34. Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, Kovac A, Philip BK, Sessler DI, Temo J, Tramer MR, Watcha M. Consensus Guidelines for Managing Postoperative Nausea and Vomiting. Anesthesia and Analgesia, 2003; 97:62– 71.
35. Cohen MM, Cameron CB, Duncan PG. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg 1990; 70: 160–7
36. Sossai R, Johr M, Kistler W, et al. Postoperative vomiting in children. A persisting unsolved problem. Eur J Pediatr Surg 1993; 3:206–208.
37. Lee, Y. Y., K. H. Kim, and Y. H. Yom. “Predictive models for post-operative nausea and vomiting in patients using patient-controlled analgesia.” Journal of international medical research 35, no. 4 (2007): 497-507.
38. Hawthorne J. (1995). Understanding and Management of Nausea and Vomiting. Oxford: Blackwell Science.
39. Chauhan, Gaurav, Deepika Madan, Kapil Gupta, Chandni Kashyap, Prashant Maan, and Pavan Nayar. “Effect of intraoperative intravenous crystalloid infusion on post-operative nausea and vomiting after diagnostic gynaecological laparoscopy: Comparison of 30 ml/kg and 10 ml/kg and to report the effect of the menstrual cycle on the incidence of...” Anesthesia: Essays & Researches 7, no. 1 (2013).
40. Matchock RL, Levine ME, Gianaros P J, Stern, RM. Susceptibility to Nausea and Motion Sickness as a Function of the Menstrual Cycle. Womens Health Issues. 2008; 328-335
41. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I et al. A Factorial Trial of Six Interventions for the Prevention of Postoperative Nausea and Vomiting. The New England Journal of Medicine. 2004, June 350(24): 2441-2451
42. Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999; 91: 693–700
43. Yotsui T. Clonidine premedication prevents sympathetic hyperactivity but does not prevent hypothalamo-pituitaryadrenocortical responses in patients undergoing laparoscopic cholecystectomy. Anesthesia J 2001;15(2):78-82.
44. Raphael JH, Norton AC: Antiemetic efficacy of prophylactic ondansetron in laparoscopic surgery: randomized, double-blind comparison with metoclopramide. Br J Anaesth 1993; 71: 845 – 848.
45. Fujii Y, Saitoh Y, Tanaka H, Toyooka H: Prevention of PONV with granisetron, droperidol or metoclopramide in patients with post-operative emesis. Can J Anaesth 1998;45: 153 – 156
46. Subramaniam, R., B. Ghai, M. Khetarpal, and M. S. Subramanyam. “A comparison of intravenous ketoprofen versus pethidine on peri-operative analgesia and post-operative nausea and vomiting in paediatric vitreoretinal surgery.” Journal of postgraduate medicine 49, no. 2 (2003): 123.
47. Rowley MP, Brown TCK. Postoperative vomiting in children. Anaesth Intensive Care 1982;10:309-13.
48. Heyland K, Dangel P, Gerber AC. Postoperative nausea and vomiting in children. Eu r J Paediatr Surg 1997;7:230-3.
49. Lonie DS, Harper NJN. Nitrous oxide, anaesthesia and vomiting: the effect of nitrous oxide anaes-thesia on the incidence of vomiting following gy-naecological laparoscopy. Anaesthesia 1986; 41: 703-707.
50. Choi, D. K., J. H. Chin, E. H. Lee, O. B. Lim, C. H. Chung, Y. J. Ro, and I. C. Choi. “Prophylactic control of post-operative nausea and vomiting using ondansetron and ramosetron after cardiac surgery.” Acta anaesthesiologica Scandinavica 54, no. 8 (2010): 962-969.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License