IJCRR - 7(1), January, 2015
Pages: 13-19
Print Article
Download XML Download PDF
EFFECT OF DIFFERENT PARAMETERS ON BIODECOLOURIZATION OF AZO REACTIVE RED RB DYE FROM TEXTILE EFFLUENT
Author: T. Madhuri, B. Lakshmi Kalyani, P. Suvarnalatha Devi
Category: General Sciences
Abstract:The aim of the present study is to isolate textile dye effluent degrading organisms. The synthetic textile dye effluents released into the environment, pollute soil and water ecosystem. Many of these effluents are toxic, carcinogenic and mutagenic in nature which effect aquatic and soil flora and fauna. Thus there is a need of processing these effluents before releasing into the environment. The Bacteria was isolated from soil contaminated with textile dye effluent by serial dilution followed by pour plating technique on nutrient agar medium. The ability of degradation was assessed by decolourization assay. Four isolates (RR1, RR2, RR3 and RR4) had the ability to degrade the dye effluent at different concentrations. The effect of pH, temperature, carbon and nitrogen sources and time course of decolourization was observed. The isolated RR2 and RR3 showed significant decolourization of dye at 600ppm.The ideal temperature was 370C and pH 7 and 9. Both isolates RR2 and RR3 showed optimum growth in media supplemented with sucrose and glucose as carbon source and RR2 showed good growth in ammonium sulphate and RR3 in peptone as a nitrogen source. The result concludes that the RR2 and RR3 isolates showed marked decolourization for textile dye effluent.
Keywords: Azo dye, Decolourization, Bioremediation, Red RB
Full Text:
INTRODUCTION
Among the textile industries, one of the most extensively used as dyes are synthetic chemicals. Approximately 10,000 different dyes and pigments are used industrially and 0.7 million tonnes of synthetic dyes produced annually, worldwide (Dawker et al., 2008; Rafi et al., 1990). India’s dye industry produces different type of dyes and pigments. Nearly 7,00,000 tonnes of dyes are produced annually Worldwide (Zollinger, 1987). Production of dye stuffs and pigments in India is close to 80,000 tonnes. India is the second large exporter of dye stuffs and intermediates after China (Mathur et al.,2005). Azo dyes being the large group of synthetic dyes constitute up to 70% of all known commercial dyes produced (Carliell et al., 1998). Synthetic dyes generally classified in to reactive, acidic, vat, dispersing, direct and sulphur etc. During the dyeing process, approximately 10-15% of the dyes are released in to the wastewaters, causing serious environmental and health hazards (Chen et al., 2003).Disposal of these dyes into the environment causes serious threat, since they may significantly affect the photosynthetic activity of hydrophtes by reducing light penetration and also toxic to aquatic organisms due to their breakdown products (Hao et al., 2000; Aksu et al., 2007). Dyes may also be toxic to some aquatic life due to the presence of aromatics and metals, chlorides etc (Gupta et al., 2003). The textile finishing generates a large amount of waste water containing dyes and represents one of the largest causes of water pollution (Maulin et al., 2013). Considerable attention has been given in evaluating the capability of microorganisms in decolourizing and degrading the azo dyes. Many studies on the decolourizing capability of microorganisms especially fungi and bacteria have been reported (Feng et al., 2012). Various physical and chemical methods have been used for the removal of dyes from wastewater effluent (Jadhav et al., 2008). However, implementation of physical and chemical methods have inherent drawbacks of being economically unfeasible, unable to completely remove the recalcitrant azo dyes and organic metabolites may cause pollution problems and involving complicated procedures (Forgaus et al., 2004; Jadhav et al., 2008).
MATERIALS AND METHODS
Dyes and characterization Dye
– Reactive red RB was chosen for decolourization in the present study and was provided by a dyeing unit, Satravada, Chittoor District of Andhra Pradesh, India.
Standard dye solution
The dyes were dissolved in sterile distilled water to a concentration of 500 mg/10 ml.
Screening and Identification of microorganisms
Dye degrading bacteria was isolated from the textile industry effluent by serial dilution method (Madigan et al., 2000). The pure isolates were maintained on nutrient agar at 40 C for further use. These isolates were streaked on Luria bertani medium amended with dye, incubated at room temperature and observed for clear zone around the colony. Among the seven isolates four showed the decolourization activity and they were selected for further use.
Decolourization studies
Decolourization activity was expressed in terms of percentage of decolourization and was determined by monitoring the decrease in absorbance at absorption maxima (λmax 518) of respective dye. This was calculated using the following formula as described by (Sani et al., 1999).
Effect of Physicochemical conditions on decolourization activity
Effect of pH and temperature
Various physical parameters like pH (5, 6, 7, 8 and 9) and temperatures (27°C, 37°C and 47°C) were monitored to study their effect on decolourization of reactive red RB dye.
Effect of dye concentration
Different concentrations of dye like 200, 400, 600, 800 and 1000ppm were incorporated into 250 ml Luria bertani medium, inoculated and incubated at optimum conditions.
Effect of differentC and N sources
Different carbon sources like glucose, sucrose, lactose and maltose were incorporated separately into Luria bertani medium at 1% concentration level. Similarly, different sources of nitrogen like yeast extract, peptone and ammonium sulphate at 0.1% concentration were added individually.
Time course of dye decolourization
The time course of decolourization was carried out under optimum conditions are: for RR2 isolate initial dye concentration at 600ppm,pH 7, 37°C, 1% sucrose , and 0.1%ammonium sulphate, for RR3 isolate at initial dye concentration at 600ppm, pH 9, 37°C, 1% glucose and 0.1% peptone. Flasks were incubated up to 36 hours at their respective temperature and samples were removed at regular intervals for every 6 hours and analyzed for decolourization activity as describe above.
STATISTICAL ANALYSIS
The experiment was done in triplicate for each parameter. The results were expressed as percentage of decolourization with respect to control values and compared by two-way ANOVA and DMRT test. A difference was considered statistically significant if p≤0.05.
RESULTS
Screening of dye degrading bacteria
The total seven isolates were isolated from textile dye effluent and screened for degradation by streaking on dye amended Luria bertani medium. Among the seven isolates four showed efficient decolourization. Then the four isolates named as RR1, RR2, RR3 and RR4 respectively. Among the four degraded bacterial isolates, RR3 showed highest decolourization activity followed by RR2, RR1 and RR4 respectively.
Decolourization studies
250 ml of Luria bertani broth amended with 600ppm concentration of red RB dye was inoculated with 4 isolates individually. All the flasks were incubated in static conditions at 370 C for 7 days. 5ml of samples was withdrawn at regular intervals and centrifuged at 10000 rpm for 10min. The supernatant was collected and the percentage of decolourization was measured at 518nm using UV-Spectrophotometer. The uninoculated dye medium supplemented with respective dye was used as blank (Jacob Thomson, 1998). Decolourization activity (%) was calculated by using the above formula and all assays were done in triplicates and the Mean value was taken for statistical analysis.
Effect of Physicochemical conditions on decolourization activity
Effect of pH and temperature
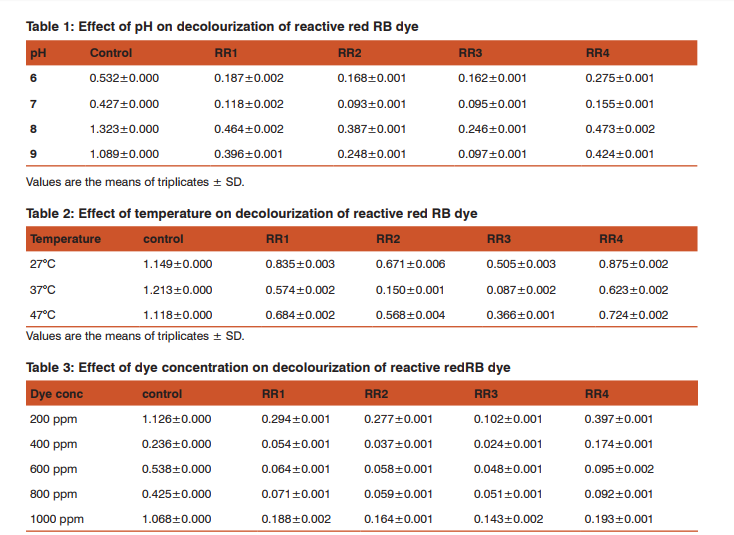
The pH exhibited varied range of effect on dye decolourization (Table 1). RR1, RR2 and RR4 isolates exhibited highest degrading capability at pH 7, whereas RR3 at pH9, respectively. The different temperatures also showed varied range of effect on dye decolourization as shown in (Table 2). All 4 isolates exhibited highest degrading capability at 37°C respectively.
Effect of dye concentration
The different dye concentration exhibited varied range of effect on dye decolourization (Table 3). RR1, RR2, RR3 and RR4 isolates exhibited highest degrading capability at 600ppm concentration.
Effect of different C and N sources
The effect of different carbon sources exhibited varied rang of effects on dye decolourization (Table 4). RR1 and RR2 isolates showed highest degrading capability in sucrose while RR3 and RR4 showed maximum growth in glucose. The different nitrogen sources also showed effect on dye degrading of dye decolourization (Table 5). RR1and RR4 isolates exhibited highest degrading capability in yeast extract, for RR2 in ammonium sulphate and for RR3 in peptone.
Time course of dye decolourization
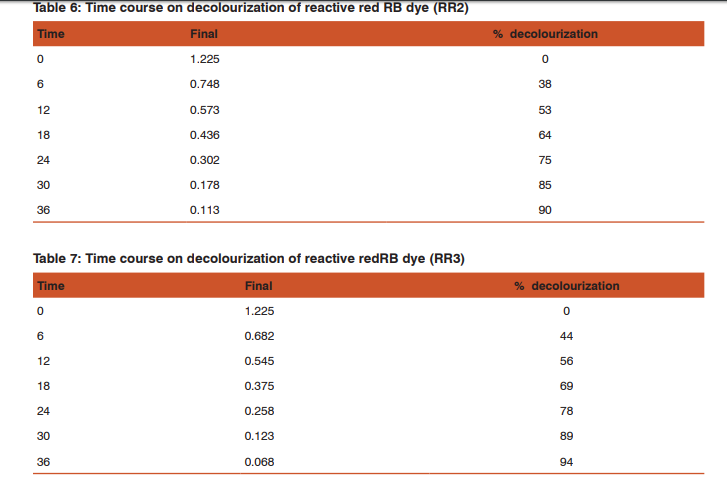
The present study reveals the high decolourization of textile dye effluent by four isolates with optimization of conditions at 84%, 90%, 94% and 80% of decolourization of dye at 36 hours.
DISCUSSION
Screening of dye degrading bacteria
The process of degradation of textile dyes by employing microorganisms particularly bacteria were also carried out to reduce environmental pollution (Srividhya et al., 2012). The present study was carried out to examine the degradation of dye by isolating the bacteria from textile dye effluents. Among the seven isolates, only four isolates showed positive results for dye decolourization, as indicated by the change and disappearance of colour of the dye from the dye-containing media of the petri plates. A zone of different decolourization around the bacterial colony which might be due to the production of extracellular enzymes by the bacteria during the biodegradation of tested dye (Joshni et al., 2011; Ajay kumar pandey et al., 2012). RR3 and RR2 isolates exhibited maximum decolourization when compared to RR1 and RR4.
Effect of Physicochemical conditions on decolourization activity
Effect of pH and temperature
The pH has a maximum effect on the efficiency of dye decolourization and the optimal pH for colour removable is between 6.0 and 10.0 for most of the dyes (Chen et al., 1999). From the above data it can be inferred that RR3 (91%) at pH 9.0 is the ideal for its activity and is more efficient in decolourizing azo dye, similarly pH plays a great influence in decolourization of Reactive Red 2 dye. These findings are closely similar with alkaline pH for Bacillus species at pH 9.0 showed decolourization percentage was 64.34% by (EI-Sersy et al., 2007). The results revealed that RR3 is more efficient in decolourizing azo dye at 37°C is the ideal temperature for its activity. Our results similar to that (Ponaj et al., 2011) were reported as the range of activity on decolourization of orange 3R with Bacillus sps at 37°C was 78.57%. Centin et al. (2006) reported that decolourizing activity was suppressed at 45°C, might be due the loss of cell viability or deactivation of the enzymes responsible for decolourization at higher temperature.
Effect of dye concentration
Maximum dye degradation was observed at 600ppm concentration for four isolates. However, when the dye concentration was high, the isolates showed less capability. Similar results were also mentioned by (Khalid et al., 2012).Dye concentration can influence the efficiency of microbial decolourization through a combination of factors including the toxicity imposed by dye at higher concentration (Sahasrabudhe et al., 2011).
Effect of different C and N sources
RR3 and RR4 exhibited maximum growth in glucose. Dyes being deficient in Carbon sources the biodegradation of dyes without any extra Carbon sources is very difficult (Senan et al., 2004). The reason for low decolourization at sucrose, fructose and maltose might be that this carbon sources could not meet the good growth requirements for the bacterial isolate. Wang et al., (2009) reported a Citrobacter sps, decolourized by 96.2% of reactive red 180 dye with 4g/l of glucose as carbon source. Bacterial utilization of azo dyes as a source of carbon and energy has been reported by (Yatome et al., 1993; Dykes et al., 1994).RR2 showed maximum in ammonium sulphate and RR3 in peptone as a nitrogen source. From the above data it was revealed that RR3 is more efficient in decolourizing azo dye with peptone is the ideal nitrogen source for its activity .The similar results was observed by (Chen et al.,1999;Liu et al., 2006). The presence of peptone regenerates NADH and this acts as an electron donor for the azo dye reduction. In addition, peptone significantly enhances the strain’s activity of azo dye decomposition and colour removal rate was increased with rise in peptone concentration (40 g l-1). The nitrogen sources were less efficient than carbon source availed by microorganism (Ola et al., 2010).
Time course of dye decolourization
The time course of decolourization of reactive red RB dye under optimum conditions was 36 hours for RR2 90%, whereas 94% of decolourization was observed in RR3 isolate within 36 hours showed (Table 6 and 7).The outcomes of this experiment indicated that RR2 and RR3 showed the decolourization process to a better extent than RR1 and RR4. However, both the species of bacteria can be inferred as good agents for the degradation of azo dye. Jothimani et al., (1998) has reported 59% dye removal from a dyeing industry effluent using pseudomonas sps.
CONCLUSION
The present study concludes that dye degrading microorganisms RR2 and RR3from an effluent contaminated site of textile dyeing industry have potential of decolourization. This observation has established that the bacteria’s are adaptive in nature and can degrade the dye contaminants. Thus, it is concluded that the bacterial isolates can be used as a good microbial source for textile waste water treatment.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in reference of this manuscript. The authors are also grateful to authors/ editors/ publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors are highly grateful to the Department of Applied Microbiology, DST-FIST, CURIE, Sri Padmavathi Mahila Visavavidyalam, Tirupati, Andhra Pradesh, India for providing Research facilities.
Values are the means of triplicates ± SD.

References:
Ajay kumar Pandey and Vinay Dubey. Biodegradation of Azo dye reactive red BL by Alcaligenes sp. AA09. International Journal of Engineering and Science 2012; 1: 54-60.
2. Aksu Z, Kilic N, Ertugrul V and Donmez G. Inhibitory effects of chromium (Vl) and Remazol black onchromium (Vl) and dye stuff removals by Trametesversicolor. Enzyme and Microbial Technology 2007;40: 1167-1174.
3. Carliell C M, Barclay S J, Shaw C, Wheatley A D and Buckley C A. The effect of salts used in textile dyeing on microbial decolourization of a reactive azo dye. Environmental Technology 1998; 19(11): 1133–1137.
4. Cetin D and DonmezG.“Decolourization of reactive dyes by mixed cultures isolated from textile effluent under anaerobic conditions” Enzymes and Microbial Technology 2006; 38: 926-930.
5. Chen K C, Hung W T, Wu J Y and Houng J Y. Microbial decolourization of azo dyes by Proteus mirabilis. Journal of Industrial Microbiology and Biotechnology 1999; 23: 686- 690.
6. Chen K C, Jane Y W, Liou D J and Sz-Chwun J Decolourization textile dyes by newly isolated bacterial strain. Journal of Biotechnology 2003; 101: 57-68.
7. Dawkar V V, Jadhav U U, Jadhav S U and Govindwar S P. Biodegradation of disperse textile dye Brown 3REL by newly isolated Bacillus sp. VUS. Journal of Applied Microbiology 2008; 105: 14-24.
8. Dykes G A, Timm R G and Von Holy A. Azoreductase activity in bacteria associated with the greening of instant chocolate puddings. Applied Environmental Microbiology 1994; 60: 3027-3029.
9. EI-Sersy N A. bioremediation of methylene blue by Bacillus thuringiensis 4G 1: application of statistical designs and surface plots for optimization. Biotechnology 2007; 6 (1): 34-39.
10. Feng J, Cerniglia C E and Chen H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front Biosciences (Elite Ed) 2012; 4: 568-586.
11. Forgacs E, Cserhati T and Oros G. Removal of synthetic dyes from wastewaters: Review on Environmental International 2004; 30: 953–971.
12. Gupta R, Gigras P, Mohapatra H, Goswami V K and Chauhan B. Microbial α-amylases: a biotechnological perspective. Process Biochemistry2003; 38:1599- 1616.
13. Hao O J, Kim H and Chaing P C. Decolourization of wastewater critical reviews. Environmental and Science Technology 2000; 30:449-505.
14. Jacob Thomson. Impact of Industries on the Ground Water Quality of Tiruppur and its Ethical implications, Ph.D. Thesis, Dept. of Zoology, University of Madras, Chennai 1998.
15. Jadhav S U, Kalme S D and Govindwar S P. Biodegradation of methyl red by Galactomyces geotrichum MTCC 1360. Intenational Biodeterioration and Biodegradation 2008; 62: 135-142.
16. Joshni T Chacko and Subramaniam K. Enzymatic degradation of Azo dyes- A Review. International Journal of Environmental Sciences 2011; 1:6.
17. Jothimani P and Prabhakaran J. “Influence of bacterial system on the decolourization of dye effluent under enrichment techniques” In: State Level seminar in Recent Developments in Applied microbiology, Tamil Nadu Agricultural University. Coimbatore 1998; 25-26.
18. Khalid A, Kausar F, Arshad M, Mahmood T and Ahmed I. Accelerated decolourization of reactive azo dyes under saline conditions by bacteria isolated from Arabian seawater sediment. Applied Microbiology and Biotechnology 2012; DOI 10.1007/s00253-012-3877-7.
19. Liu G, Zhou J, Wang J, Song Z and Qv Y. Bacterial decolourization of azo dyes by Rhodo pseudomonas palustris, World Journal of Microbiology and Biotechnology 2006 ;22: 1069-1074.
20. Madigan M T, Martinko J M, Parker J and Brock. Biology of microorganisms, Ed. Prentice Hall, New Jersey, USA 2000.
21. Mathur N, Bathnagar P, Nagar P and Bijarnia M K. Mutagenicity assessment of effluents from textile/dye industries of Sanganer, Jaipur (India): a case study. Ecotoxicological Environmental safety, 2005; 61:105-113.
22. Maulin P, Shah Patel K A, Nair S S and Darji A M. Bioremoval of Azo dye Reactive Red by Bacillus spp. ETL-1982. Journal of Bioremediation and Biodegradation 2013; 4: 3.
23. Ola I O, Akintokun A K, Omomowo I O and Areo V O. Aerobic decolourization of two reactive azo dyes under vary ing carbon and nitrogen source by Bacillus cereus, African Journal of Biotechnology 2010 ; 9(5) : 672-677.
24. Ponraj M, Gokila K and Zambare V. Bacterial decolourization of textile dye- Orange 3R. International Journal of Advanced Biotechnology and Resources 2011; 2: 168-177.
25. Rafii F, Franklin W and Cerniglia C E. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Applied Environmental and Microbiology1990; 56: 2146-2151.
26. Sahasrabudhe M and Pathade G. Decolourization of C.I. Reactive Yellow 145 by Enterococcus faecalis strain YZ66. Journal of Science and Resources 2011; 3(3):403-414.
27. Sani R K and Banerjee U C. Screening for organisms applicable to the decolourization of trimethylaniline dyes and optimization of biotranformation conditions in stirred tank reactor. Indian journal of Environment Eco planning 1999; 2: 1-9.
28. Senan R C and Abraham T E. Bioremediation of textile azo dyes by aerobic bacterial consortium. Biodegradation 2004; 15(4): 275 - 280.
29. Srividhya M R, Mary L H, Goel A M and Rangabhashiyam S. “Decolourization study of synthetic optilan red dye by Aspergillusniger”, International Journal of Recent Scientific Research 2012; 11: 301-303.
30. Wang H, Su J Q, Zheng X W, Tian Y, Xiong X J and Zheng T L. Bacterial decolourization and degradation of the reactive dye Reactive Red 180 by Citrobactersp. CK3q. International Bio deterioration and Biodegradation 2009; 63: 395-399.
31. Yatome C, Matsufuru H, Taguchi T and Ogawa T. Degradation of 4 dimethylaminobenzene-2-carboxylic acids by Pseudomonas stutzeri. Applied Microbiology Biotechnology1993; 39: 778-781.
32. Zollinger H. Colour Chemistry: Synthesis, Properties and Applications of Organic Dyes and Pigments VCH Newyork, 1987; 92-102.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License