IJCRR - 7(4), February, 2015
Pages: 54-59
Print Article
Download XML Download PDF
PROTEIN ENERGY WASTING (PEW) / CACHEXIA IN CHRONIC KIDNEY DISEASE - ROLE OF LEPTIN AND INSULIN
Author: D. Ponnudhali, P. Nagarajan, R. Shankar
Category: Healthcare
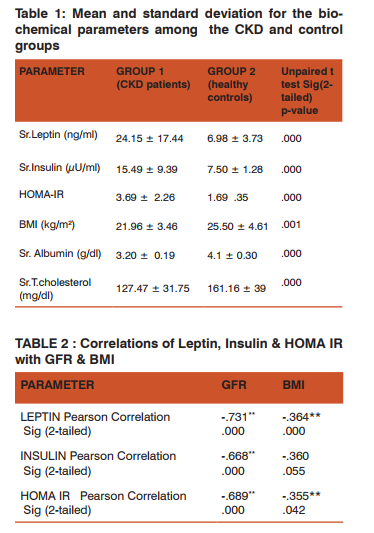
Abstract:Background: The prevalence of protein energy wasting (PEW)/ cachexia is very high among patients with chronic kidney disease (PEW), increasing in severity with the progression of the disease. Among other factors, increased serum Leptin levels and insulin resistance have been implicated in the pathogenesis of PEW/cachexia in CKD Aim and objectives: To assess the serum leptin & insulin levels along with HOMA IR (Homeostasis model assessment-Insulin Resistance) in non diabetic chronic kidney disease patients and to study the correlation of these parameters with glomerular filtration rate (GFR) & body mass index (BMI). Materials and methods: Non-diabetic CKD patients (group1; n=45) and healthy non-diabetic individuals (group 2; n=45) with normal renal function were recruited for the study. Serum leptin and Insulin levels were assessed using ELISA kits. Calculated values of HOMA IR & BMI were taken for the analyses.Statistical analysis: The statistical analysis was done using SPSS version 16. The parameters were compared among the 2 groups using Independent t test and correlation coefficient. Results: Serum Leptin levels (24.15 \? 17.44 ng/ml) were increased significantly (p=.000) in group 1 patients compared to those in group 2 (7.50 \? 1.28 ng/ml). Serum insulin levels (p=.000) were increased in CKD patients (15.49 \? 9.39 \?U/ml) from that of the healthy controls (7.50 \? 1.28\?U/ml). The HOMA IR was also significantly high (p=0.000) in the CKD group ( 3.69 \? 2.26) than the controls (1.69 \? 0.35). Leptin, insulin & HOMA IR showed a highly significant negative correlation with GFR & BMI .The serum albumin and total cholesterol in the CKD group were 3.20 \? 0.19 g/dl & 127.47 \? 31.75 mg/dl respectively. Conclusion: Hyperleptinemia and insulin resistance may be responsible for protein energy wasting/cachexia associated with CKD.
Keywords: Leptin, protein energy wasting (PEW), Cachexia, Insulin resistance
Full Text:
INTRODUCTION
Protein energy wasting (PEW)/ cachexia is highly prevalent among patients with chronic kidney disease(CKD). PEW is a devastating complication of CKD, as it increases the incidence of cardiovascular outcomes as well as the morbidity & mortality associated with CKD, making it a major clinical problem. The International Society of Renal Nutrition and Metabolism (ISRNM) panel has described PEW as a state of decreased body stores of protein and energy fuels (body protein and fat masses). It describes a progressive loss of adipose tissue and lean body mass, with cachexia constituting the severe form of protein energy wasting (PEW) [1].The PEW/cachexia syndrome in CKD patients consists of anorexia, increased energy expenditure, decreased protein stores characterized by low serum albumin, loss of body weight and loss of muscle mass. The etiology of PEW in chronic kidney disease (CKD) is complex and includes inflammation, metabolic acidosis, insulin resistance, increase in serum leptin [2], transient catabolic illnesses, hyperparathyroidism and so many other conditions. CKD is associated with insulin resistance even from an early stage, when the GFR is normal [3]. Leptin is an adipocyte- derived hormone that has an inhibitory effect on food intake while it increases the energy expenditure. Elevated levels of serum leptin have been reported in CKD patients and has been linked with the pathogenesis of PEW/cachexia [4,5]. Hence we decided to assess the serum leptin & insulin levels along with HOMA- insulin resistance, in non diabetic CKD patients, graded to have PEW/ cachexia. We have also correlated the levels of these parameters with glomerular filtration rate(GFR) and body mass index(BMI). This study is an attempt to highlight the role played by leptin and insulin in the pathogenesis of PEW in non diabetic CKD and, its relation to the declining GFR & BMI.
MATERIALS AND METHODS
Our study has been conducted in patients with chronic kidney disease who were non diabetic and not on dialysis, recruited from the department of Nephrology, Government Mohan Kumaramangalam Medical College Hospital, Salem. Friends, family members and colleagues of the patients constituted the control group. Written consent was obtained from all the subjects, after clearly explaining to them about our study protocol. This study was approved by the ethical committee of Govt Mohan Kumaramangalam Medical College Hospital. Group 1 comprised of 45 non diabetic patients with Chronic Kidney Disease (CKD), diagnosed and staged, based on NKF K/DOQI guidelines [6]. Group 2 comprising of 45 healthy adults with normal renal function (GFR > 90 ml/min), formed the control group. The study group 1 included CKD patients, not on hemodialysis/ peritoneal dialysis. Patients with history of diabetes, metabolic syndrome, endocrine disorders, obesity, pregnancy, malignancy or any other terminal illness were excluded from the study. The control group 2 included healthy individuals, with normal renal function. Subjects with diabetes, hypertension, renal disease, chronic infections, pregnancy, systemic illness, endocrine disorders, malignancy or neuropsychiatric illness were excluded from the study. All subjects in both the control and study group were age and sex matched.
Laboratory analysis:
After an overnight fast of at least 8 hrs, fasting blood samples were collected from both the patients and controls. Plasma and serum were separated immediately after collection, and stored at -20°C , until further analysis. Complete blood count, urine routine , blood glucose, urea, creatinine, albumin & Total Cholesterol were estimated using the semi autoanalyzer- Microlab 300. These biochemical analyses were done, in the clinical Biochemistry Laboratory, VMKV Medical College, Salem. Serum Leptin was analyzed using DRG (sandwich) EIA 2395 ELISA kit [7]. Serum Insulin was analyzed using Monobind’s Insulin/ MAPS ELISA kit [8]. Homeostasis model assessment-Insulin Resistance (HOMA-IR) was calculated using the formula, HOMA-IR = fasting serum insulin (µIU/ml)* fasting plasma glucose (mg/ dl)/405 [9]. GFR was calculated using the MDRD (Modification of Diet in Renal Disease) formula available online [10].
PROTEIN ENERGY WASTING (PEW)/ CACHEXIA:
We have tried to assess the protein energy wasting (PEW) in the CKD patients based on the following criteria, proposed by The International Society of Renal Nutrition and Metabolism (ISRNM):


We have also calculated BMI using the formula: Weight in kilograms / (height in meters)2 Kg/m2 [12].The standard weight status categories associated with BMI ranges for adults are Herewith we have selected 4 parameters to diagnose the protein energy wasting (PEW) in CKD patients - serum albumin, serum cholesterol, BMI & Dietary Energy Intake- DEI. Nutritional diaries were provided to the CKD patients and they were taught to make entries, which will explain their complete dietary regimen through out the day. From the entries their dietary energy intake was calculated, for further analysis.
Statistical analysis
Statistical analysis was done using the software SPSS version 16. Data was expressed as mean ± 2SD and differences in mean between the 2 groups were analyzed using independent t test. Bivariate correlations of Leptin, Insulin & insulin resistance (IR) with BMI and GFR, were performed using Pearson’s correlation.
RESULTS
The present study was conducted in 45 non diabetic CKD patients (group1) not on dialysis and 45 healthy controls (group2), with normal renal function. Serum leptin, insulin, albumin and total cholesterol were analyzed in the blood samples. HOMA IR and BMI were the calculated parameters, taken for the study. The results are displayed in Table 1. Serum Leptin levels (24.15 ± 17.44 ng/ml) were increased significantly (p=.000) in group 1 patients compared to those in group 2 (7.50 ± 1.28 ng/ml). Serum insulin levels (p=.000) were increased in CKD patients (15.49 ± 9.39 µU/ml) compared to the healthy controls (7.50 ± 1.28µU/ml). The HOMA IR was also significantly high (p=0.000), in the CKD group. The correlations of serum Leptin, Insulin & HOMA-IR with BMI & GFR, were performed using Pearson’s correlation which is depicted in Table-2. Leptin, Insulin & HOMA IR were found to have a highly significant negative correlation with GFR & BMI . Indicators of protein energy wasting/ cachexia: Certain biochemical tests were done to assess the status of protein energy wasting/ cachexia. Serum albumin (3.20 ± 0.19g/dl) was found to be significantly decreased (p=.000) in the group 1 patients from that of the group2 (4.1 ± 0.30 mg/dl) subjects. Serum cholesterol levels (group1: 127.47 ± 31.75mg/dl, group2: 161.16 ± 39 mg/dl) showed a significant decrease in group1 patients (p=.000). The BMI levels were significantly decreased (p=.001) in the group1 (21.96 ± 3.46 kg/m2) patients compared to those in group 2 (25.50 ± 4.61 kg/m2). We have found that the serum albumin levels were below 3.8 g/dl in the CKD patients. The serum cholesterol levels though were decreased in CKD patients , their levels were not below 100mg/dl. The BMI levels were below 23 in the CKD patients. All these values are shown in Table 1. The dietary energy intake (DEI) of CKD patients was assessed from their nutritional dairies. Their mean DEI was found to be less than 24 kcal kg−1day−1 . The CKD patients in group 1 satisfy three of the four criteria stated by the ISRNM [11]. Hence the patients in group 1, can be placed under the category of protein energy wasting (PEW)/ cachexia syndrome.
DISCUSSION
We have conducted our study in 45 non diabetic predialysis CKD patients and 45 healthy controls. Serum leptin levels have been significantly increased in CKD patients (group1) compared to healthy controls (group 2). There is negative significant correlation of serum leptin levels with BMI & GFR. Leptin is a 167 amino acid peptide , an adipocytokine, produced abundantly by the adipose tissue and acts as a major regulator of food intake and energy homeostasis. It circulates as both free and protein- bound form and exerts inhibitory effects on food intake while increasing energy expenditure [13]. The leptin receptor belongs to class I cytokine receptor superfamily and possesses strong homology to the signal transducing subunits of the IL-6 receptors [14]. At least five isoforms of receptors (OBRa, OBRb, OBRc, OBRd, and OBRe) are known to exist and result from alternate gene splicing. Among these most biological effects of leptin are mediated by the leptin receptor OBRb, which is primarily present in the hypothalamus, where action of leptin is important in energy homeostasis. The OBRb receptors are also expressed in peripheral tissues including heart, skeletal muscle , adrenals, kidneys, adipocytes , smooth muscle cells, endothelial cells etc . An association of increased serum leptin levels with CKD, have been emphasized in certain studies, in different populations. [15,16,17]. Leptin is cleared from the circulation by kidney, by glomerular filtration followed by metabolic degradation in the renal tubules [17]. In CKD, due to reduced GFR, there is increased serum leptin levels. We have also found a negative significant correlation between serum leptin levels and GFR , which confirms that serum leptin is increased due to declining renal function in CKD. In CKD, inflammation is another important factor which contributes to hyperleptinemia [13]. The increased leptin levels may mediate protein energy wasting and cachexia, by regulating food intake and energy consumption via signalling through the hypothalamic melanocortin system [18]. Pro-opiomelanocortin (POMC) is a propeptide precursor that is produced in neurons found in the hypothalamic arcuate nucleus [19]. POMC neurons are thought to provide tonic inhibition of food intake and energy expenditure by production and release of α-melanocyte stimulating hormone (α- MSH) [4]. α- MSH in turn activates the hypothalamic type 4 melanocortin receptor (MC-4R), leading to suppressed food intake and increased energy expenditure. Leptin is able to activate the POMC neurons in the hypothalamus, triggering the production and release of α- MSH, which binds to MC3/MC4 receptors expressed on the hypothalamic nuclei, inducing a reduction of appetite and increase in energy consumption [20]. Leptin also suppresses the activity of Neuropeptide Y & Agoutirelated peptide (AGRP), which are endogenous antagonists of MC- 4R [21]. Hence increased serum leptin levels could be an important causal factor for protein energy wasting/ cachexia seen in CKD patients [4,5].This fact has been tested by Wai Cheung et al, who have identified that leptin causes MC-4R blockade and that it plays a significant role in transducing cachexic signals in CKD [4]. Pecoits- Filho et al suggest that free circulating leptin concentrations are elevated in patients with end stage renal disease and may be associated with inflammation associated cachexia [5]. As we have observed in our study, serum leptin increases as the BMI decreases, showing a probable relation between increasing leptin levels and PEW/ cachexic manifestations. Hence leptin may play an important role in anorexia/ cachexia syndrome seen in CKD patients. Some of the causes of protein energy wasting (PEW) in CKD patents are inflammation, metabolic acidosis and insulin resistance [21]. Leptin secretion have been found to be regulated by insulin, glucocorticoids and catecholamines [22]. In our study we have identified the occurrence of hyperinsulinemia and increased HOMA-IR, in CKD patients. Hyperinsulinemia may be due to reduced clearance of insulin by the kidneys / compensatory to insulin resistance. We have also obtained negative significant correlation between serum insulin levels & HOMA-IR with GFR and BMI. The insulin resistance in CKD, results in uremic myopathy due to increased muscle breakdown. Insulin has an anticatabolic effect, by activating protein synthesis and inhibiting proteolysis. In insulin resistance, there is decreased utilization of glucose as an energy source, by the skeletal muscles.CKD attenuated insulin stimulated protein synthesis and increased protein degradation in skeletal muscle [23]. Many studies have documented insulin resistance as an important complication of CKD, with varied metabolic changes [24,25,26]. In CKD, insulin resistance is due to a post-receptor signalling defect : reduced activity of PI3K (phoshpatidylinositol 3-kinase) in turn causing reduced levels of phoshorylated Akt (pAkt). This dysfunction of PI3K signalling pathway is a common initiator of muscle protein degradation by enhancing the activity of ubiquitin-proteosome pathway, in muscle [27]. Ubiquitin proteosome proteolytic (Ub-P’some) system consists of the 7- KDa protein ubiquitin, E3 ubiquitin ligases (atrogin-1, MAFbx & MuRF1) and proteosome (large multi-subunit complex found in all mammalian cells). Insulin resistance activates the Ub-P’some system through suppressing PI3 Kinase pathway & by activating MEK/ERK pathway [28]. This PI3K signalling defect activates FOXO group of transcription factors ,which in turn induces the expression of ubiquitin conjugating enzymes atrogin-1, MAFbx & MuRF1 [29]. Insulin resistance also results in the activation of MEK/ERK pathway, causing increased expression of ubiquitin (UbC) . Hence the Ubiquitin proteosome proteolytic (Ub-P’some) system is activated as shown in figure1. The suppression of PI3K signalling pathway also results in activation of Bax proteins which in turn activates the enzyme caspase 3. Caspase 3 plays an initial role in muscle protein degradation, by cleaving actomyosin and presenting them to the ATP dependent Ubiquitin proteosome proteolytic (Ub-P’some) system which in turn degrades the monomeric actin/myosin fragments but not the actomyosin complexes [30]. Hence increased insulin resistance seen in CKD patients may play an important role in muscle protein degradation causing a reduction in lean body mass. An important consequence of insulin resistance in CKD is the pathogenesis of PEW/ cachexia [31]. Leptin and insulin- Role in kidney damage: Serum leptin levels and insulin resistance increases in chronic kidney disease and both these parameters play an important role in the pathogenesis of PEW/ cachexia. Apart from this both leptin & insulin are known to activate the sympathetic nervous system, causes impairment of natriuresis

and inhibition of nitric oxide synthesis [32,33]. All these factors may contribute to the up regulation of blood pressure and hence worsening of renal function.
CONCLUSION
The present study shows an increase in serum leptin, insulin and HOMA-insulin resistance, showing a strong correlation with GFR & BMI. Increased leptin signalling and insulin resistance might significantly contribute to the development of protein energy wasting (PEW)/ cachexia syndrome in patients with chronic kidney disease. Understanding the role played by these parameters might help in early intervention of the wasting disorder, when the skeletal muscle complications might still be reversible. The emerging role of therapeutic agents like AGRP (agouti-related peptide- endogenous antagonist of MC-4R receptors) & orexigenic agents/appetite stimulants (neuropeptide Y, Ghrelin) and their appropriate use in maintaining the skeletal muscle homeostasis, should be further confirmed by well controlled studies.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed
References:
1. Fouque D, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int.2008;73:391-8.
2. Sarraf P, Frederich RC, Turner EM et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia.J Exp Med 1997;185:171- 175
3. Eidemak I, Feldt-Rasmussen B, Kanstrup IL et al. Insulin resistance and hyperinsulinemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia.1995; 38: 565–57.
4. Wai Cheung, Pin X. Yu, Brian M. Little, Roger D. Cone, Daniel L. Marks, and Robert H. Mak. Role of leptin and melanocortin signalling in uremia-associated cachexia. J. Clin. Invest. 2005;115 :1659–1665.
5. Pecoits-Filho R, Nordfors L, Heimburger O et al. Soluble leptin receptors and serum leptin in end-stage renal disease: relationship with inflammation and body composition. Eur J Clin Invest. 2002; 32: 811–817.
6. RateGraham RD Jones and Ee-Mun Lim. The National Kidney Foundation Guideline on Estimation of the Glomerular Filtration.Clin Biochem Rev. Aug 2003; 24(3): 95–98.
7. Nazish Rafique, Mohammad Nasir Afzal. Relationship of serum leptin levels with body mass index and gender . RMJ. 2009; 34(2): 164-166.
8. A Piyali das et al. Association of obesity and leptin with insulin resistance in type 2 diabetes mellitus in indian population. Indian J Physiol Pharmacol. 2013; 57(1): 45–50.
9. Ali Movahed et al. Antihyperglycemic Effects of Short Term Resveratrol Supplementation in Type 2 Diabetic Patients. Evidence-Based Complementary and Alternative Medicine. Volume 2013, 1-11.
10. Wieneke Marleen Michels et al. Performance of the Cockcroft-Gault, MDRD, and New CKDEPI Formulas in Relation to GFR, Age, and Body Size. Clin J Am Soc Nephrol. 2010; 5:1003–1009.
11. Yashpal P. Jadeja, Vijay Kher. Protein energy wasting in chronic kidney disease: An update with focus on nutritional interventions to improve outcomes Indian Journal of Endocrinology and Metabolism / Mar-Apr 2012 / Vol 16 | Issue.
12. Kronenberg. F et al. Lipoprotein(a) serum concentrations and apolipoprotein(a) phenotypes in mild and moderate renal failure. J Am Soc Nephrol. 2000;11: 105–1
13. RH Mak, W Cheung, RD Cone and DL Marks. Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney International .2006; 69:794–797.
14. G. H. Lee, R. Proenca, J. M. Montez et al. “Abnormal splicing of the leptin receptor in diabetic mice.” Nature.1996; vol 379,(6566): 632–635.
15. Relationship between Plasma Leptin Level and Chronic Kidney Disease. Anoop Shankar, Shirmila Syamala, Jie Xiao, and Paul Muntner. International Journal of Nephrology.Volume 2012; Article ID 2 69532, 6 pages.
16. Cumin F, Baum HP, De Gasparo M, Levens N. Leptin is cleared from the circulation primarily by the kidney. Int J obes Relat Metab Disord.1997;21:495–504.
17. Sharma K, Considine RV, Michael B et al. Plasma leptin is partly cleared by the kidney and is elevated in hemodialysis patients. Kidney Int.1997; 51:1980–1985.
18. Pinto, S., et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004; 304:110–115.
19. Schwartz, M.W., et al. Central nervous system control of food intake. Nature.2000; 404: 661–671.
20. M. G. Castro and E. Morrison, “Post-translational processing of pro- opiomelanocortin in the pituitary and in the brain,” Critical Reviews in Neurobiology.1997; vol 11, no 1: 35–57.
21. Mak RH. Insulin resistance but IGF-I sensitivity in chronic renal failure. Am J Physiol 1996; 271: F114– F119.
22. P. Leroy, S. Dessolin, P. Villageois et al., “Expression of ob gene in adipose cells: regulation by insulin,”Journal of Biological Chemistry.1996; vol. 271(5): 2365–2368.
23. May RC, Kelly RA, Mitch WE. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia: The influence of metabolic acidosis. J.Clin Invest 1987;79: 1099-1103.
24. Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T.Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis.2005; 45:275–280.
25. Jie Du et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Invest. 2004;113: 115–123
26. James L. Bailey et al. Chronic Kidney Disease Causes Defects in Signaling through the Insulin Receptor Substrate/ Phosphatidylinositol 3-Kinase/Akt Pathway: Implications for Muscle Atrophy. J Am Soc Nephrol.2006;17: 1388– 1394.
27. Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med.1996; 335:1897–1905.
28. Bin Zheng et al. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010;24: 2660 –2669.
29. LeeSW, DaiG ,HuZ, WangX, DuJ, MitchWE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol.2004;15:1537–1545.
30. Solomon, V., and Goldberg, A.L. Importance of the ATPubiquitin- proteasome pathway in degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J. Biol. Chem. 1996;271:26690–26697.
31. Slew ED, Ikizler TA. Dietary concerns in advanced chronic kidney disease: insulin resistance and protein energy metabolism in advanced chronic kidney disease. Semin Nephrol. 2007; 23:378 – 82. 32. N. Werner and G. Nickenig, “From fat fighter to risk factor: the zigzag trek of leptin,”
Arteriosclerosis, Thrombosis, and Vascular Biology.2004; vol. 24(1): 7–9.
33. Komers R, Anderson S. Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol. 2003; 284(6):F1121-37.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License