IJCRR - 7(4), February, 2015
Pages: 20-26
Print Article
Download XML Download PDF
Association of Serum Uric Acid with anthropometric, HbA1c and Lipid profile in Diabetic Retinopathy
Author: Munilakshmi U., Prabhavathi K., Shashidhar K. N., Madhavi Reddy, Lakshmaiah V.
Category: Healthcare
Abstract:Introduction: Diabetic Retinopathy (DR), one of the leading cause of visual impairment in adults, a kind of serious microvascular complication of Diabetes Mellitus. It is well known that purine metabolites are strongly associated with the development of diabetic microvascular complications. Uric acid, an end product of the purine metabolism, acts as a pro-oxidant and it may thus be a marker of oxidative stress.
Objectives:
1. To Estimate and Compare Anthropometric and Biochemical parameters in Diabetic Retinopathy patients, Diabetes without Retinopathy and clinically proven healthy controls.
2. To correlate Serum Uric acid levels with Anthropometric and Biochemical indices in Diabetic Retinopathy patients. Materials and Methods: Study group consisted total of 150 subjects divided into three groups- Group I (Clinically proven healthy controls), Group II (Type 2 Diabetes Mellitus without retinopathy) and Group III (Diabetic retinopathy), visiting RL Jalappa hospital and Research centre Kolar. Anthropometric & Biochemical parameters were estimated by standard methods.
Results: Comparison of Anthropometric & Biochemical parameters were done among the three Groups, we observed Age, Obesity index, Fasting Blood Sugar, HbA1c, Total Cholesterol, high density lipoproteins, low density lipoproteins & Uric acid were statistically significant with p value < 0.05 and also positive correlation was observed for Body mass index,Fasting Insulin, Total Cholesterol, high density lipoproteins and low density lipoproteins with Uric Acid in Group III.
Conclusion: Hyperglycemia and Oxidative stress in Type 2 Diabetes Mellitus leads to micro and macrovascular complications. In Group III, there was positive correlation of serum Uric Acid with Anthropometric & Biochemical Indices. Therefore uric acid can be considered as a reliable marker which is less expensive and helps clinicians in controlling the progression of DM to microvascular complications like DR.
Keywords: Diabetic Retinopathy, Diabetes Mellitus, Uric Acid, Obesity index
Full Text:
INTRODUCTION
Diabetes Mellitus a metabolic syndrome, characterized by hyperglycemia due to an absolute or a relative deficiency of insulin.Type 2 Diabetes Mellitus (T2DM) is undoubtedly one of the most challenging health problems in the 21st century and the number of diabetic patients diagnosed has reached 366 millions in 2011.Complications of diabetes areone of the major cause forreducing quality of life, disability and death. Approximately 25% of the people with newly detected diabetes already have microvascular disease, suggesting that they have had the disease for 4–7 years by the time of the diagnosis1 . In these patients, with earlier disease identification and the intensive treatment of hyperglycemia, the risk of developing microvascular complications can be reduced,particularly, diabetic retinopathy (DR)2 . Studies conducted by Klein. R et.al., stated that in age group of 30-65 years, Diabetic retinopathyis one of the leading cause for visual impairment is due to uncontrolled or long duration diabetes3 . Even today, the diagnosis of retinopathy depends on opthalmoscopy and fluorescein angiography. However, it is generally acknowledged that only the pathological changeswhich occur at the severe stages of the retinopathy canbe discovered using this diagnostic method. Thus, itis of tremendous importance to find a diagnostic marker thatcan be used for screening and prediction of retinopathy, especiallywith high precision and which should be easily measured3 . According to Ames BNet.al.,purine metabolites are strongly associated with the development of diabetic microvascular complications4 . Uric acid (UA), an end product of the purine metabolism, acts as a pro-oxidant and may thus thought to be a marker of oxidative stress. In diabetic patients, superoxide plays an important role in microvascular dysfunction and exerts direct tissue damage which leads to lipid and protein peroxidation.Uric acidhas also beenthought to have a therapeutic role as an antioxidant4 .Studies done by Nakagawa T et al., reported that hyperuricaemia has been added to the set of metabolic abnormalities which are associated with insulin resistance and/or hyperinsulinemia in the metabolic syndrome5 . UA was previously used to be thought as a predominant predictor of gouty diathesis6 . However, as a marker of metabolic syndrome (MetS), UA could worsen insulin resistance by disturbing insulin-stimulated glucose uptake and shows positive association between the serum uric acid levels and the development of T2DM7 . Studies conducted by Cirillo. P et al, Anwar M.M et al,andTanemoto M et al., reported that the high level of uric acid was associated with diabetic microvascular complications, such as nephropathy, retinopathy, and neuropathy 8,9,10, but it is usually considered a marker of tissue dysfunction rather than a risk factor for progression. Some researchers considered that uric acid might affect the function of vascular smooth muscle cells, which is related to diabetic retinopathy11. Therefore, the present study was designed to look for any association of serum uric acid with HbA1c andlipidprofile in T2DM, taking into consideration the relevant clinical, biochemical and the anthropometric data
Objectives
1. To Estimate and Compare Anthropometric and Biochemical parameters in Diabetic Retinopathy patients, Diabetes without Retinopathy and clinically proven healthy controls.
2. To correlate Serum Uric acid levels with Anthropometric and Biochemical indices in Diabetic Retinopathy.
Materials and Methods
The present study was conducted in R L Jalappa hospital attached to Sri Devaraj Urs Medical College, Kolar. Total 141individuals with the age group of 40-60 years of bothgenderswere selected in the Ophthalmology outpatient department during the year October 2011 to January 2012. These subjects were grouped into three categories Group I: Forty three clinically proven healthy controls. Group II: Forty eight T2DM subjects without retinopathy based on fundoscopic changes Group III: Fifty Diabetic retinopathy subjects based on fundoscopic examination. The study was approved by the institutional ethical clearance committee and a written informed consent was obtained from all the subjects who were enrolled in our study. Patients with hepatic disease, type 1 diabetes mellitus, peripheral vascular disease, acute or chronic infection, cancer and complications related to diabetes like ulcers, nephropathy and neuropathy, which might affect the estimation of various biochemical parameters, were excluded from the study. Clinical details such as anthropometric measurementsof all the subjects enrolled in the study were obtained from the hospital medical records. Venous blood sample was collected under strict aseptic conditions with a minimum of 8 hours of fasting.All the parameters were estimated using Johnson and Johnson vitros 250 dry chemistry auto analyzer which works on the principle of reflectance photometry. The blood glucose estimation was done by Glucose Oxidase Peroxidase method (GOD-POD)12, HbA1c was estimated by HPLC method12, Fasting Insulin by Chemilumuniscence assay12, uric acid was estimated by uricase method13 Total cholesterol (TC) was estimated by cholesterol oxidase method14, Triglycerides (TG) estimation was by Enzymatic colorimetric test- GPO PAP14, Highdensity lipoproteins (HDL) estimation was done by Direct Enzymatic method12, LDL-cholesterol, Non-HDL-cholesterol, were calculated14,15. Statistical analysis was carried out by one way analysis of variance (ANOVA) by using the SPSSversion 16.0, and p value < 0.05 wasconsidered significant and Pearsons Correlation Coefficient was used to rank different variables either positively or inversely correlated.
Results
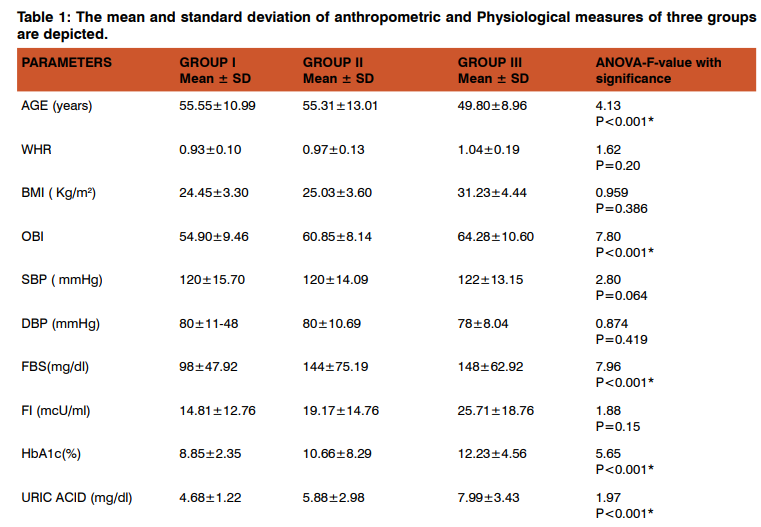
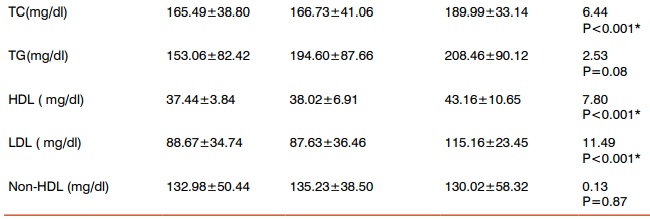
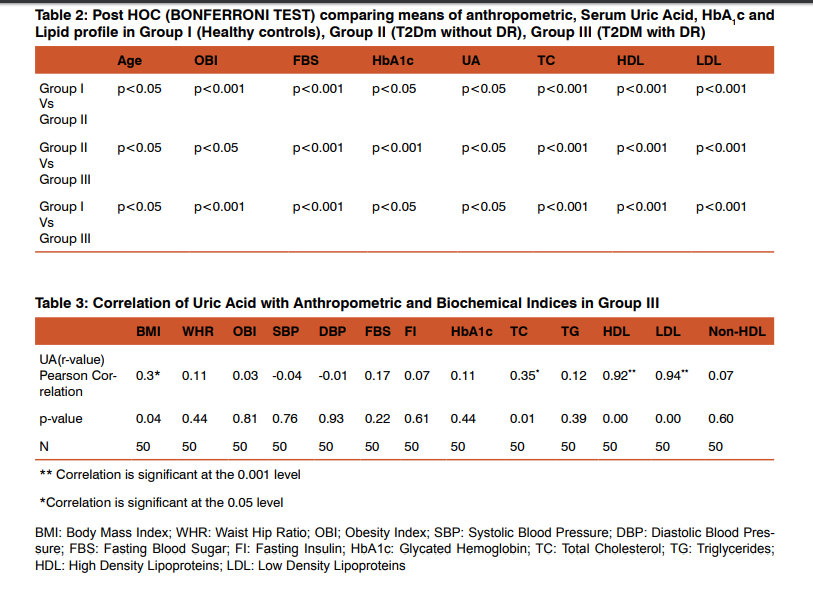
Table 1 shows the comparison of mean, standard deviation of anthropometric, physiological and biochemical indices between the three groups, age, obesity index, FBS, HbA1c, uric acid, TC, HDL and LDL-cholesterol levels were significantly higher in all the groups compared to controls. Table 2 shows the Post Hoc analysis using Bonferroni criterion for significance between Group I Vs Group II, age, OBI, FBS, HbA1c, uric acid, TC, HDL, LDL were significant (p<0.05, p<0.001). With respect to Group II Vs Group III, age, OBI, FBS, HbA1c, uric acid, TC, HDL and LDL levels were highly significant (p<0.001). On comparison between Group I Vs Group III, age, OBI, FBS, HbA1c, uric acid, TC, HDL and LDL were significant (p<0.05). Table 3 shows the Pearson’s correlation coefficient of uric acid with anthropometric, Physiological and Biochemical parameters in DR group (Group III), among these parameters BMI(r=0.03, p=0.04), TC (r=0.35, p=0.01), HDL (r=0.92, p=0.001) and LDL (r=0.94, p=0.001) cholesterol levels showed significance with strong positive correlation.
Discussion
Type 2 Diabetes represents one of the most detrimental diseases and significant public health problems due to its high incidence and prevalence as well as high risk of macro- and microvascular complications. DR, a sight threatening microvascular complication of diabetes, is the leading cause of blindness and visual dysfunction in the working age population in developed countries16. The development of DR in type 2 diabetes patients was associated with baseline glycemia, glycemic exposure over several years, poor lipid control, higher blood pressure, and smoking17. In the present study, WHR, BMI and OBI were higher in Group III compared to Group I and Group II. Study done by Oliveira E P et al., observed similar findings and reported according to the gender, the main predictors for UA increase were BMI and muscle mass for men. Waist circumference, creatinine, and muscle mass (positively); and HDL-c (negatively) were associated for women18. In the present study the mean blood pressure values was within the reference range which is consistent with the United Kingdom Prospective Diabetes Study (UKPDS) and Appropriate Blood Pressure Control in Diabetes (ABCD Study) and stated that strict blood pressure control can prevent and/or limit the development and progression of DR and visual dysfunction19,20. In the present study, serum insulin level of Group III was higher than that of Group I & Group II, however no significant difference was observed. The reason for this finding could be that the insulin resistance occurs when the cells become less sensitive to the effects of insulin. This results in rising blood sugar levels (hyperglycaemia) and a drop in the energy production. To compensate for the insulin resistance and to keep the blood glucose levels from spiraling out of control the pancreas tries to restore the balance by producing more insulin. If this isleft unchecked, the cells become even more resistant to insulin, even as the pancreas secretes ever greater amounts of insulin, in a desperate attempt to bring the system back under control, this results in dangerously high blood levels of insulin (hyperinsulinaemia). If this is not corrected, the pancreas eventually becomes exhausted, resulting in diabetes20.Similar results were reported by G SrinivasaNageswara Rao et al.,21 and Pagano G et al.,22. In our study we observed mean HbA1c values were significantly higher in Group II and Group III compared to Group I,indicating thatHbA1c has special affinity for oxygen there by causes tissue anoxia and plays a role in causation of micro and macroangiopathy, our findings were consistent with studies conducted by Correa Z et al., and Ishrat K et al.,22,23. Our study showed significant increase in mean serum uric acid levels in Group III compared to Group I & Group II. Similar results were observed by Butturini U et.al.25, this indicates that the uric acid levels have a tendency to increase with the onset of retinopathy. Uric acid has some physiologic functions including activation of the rennin angiotensin system and direct actions on endothelial cells and vascular smooth muscle cells. The retina is highly susceptible to oxidative stressbecause of intense exposure to light and oxygen and its high polyunsaturated fatty acid (PUFA) content that is prone to lipid peroxidation26. These oxidation products are toxic to the microvascular walls and therefore, may have a causal role in diabetic microvascular damage and also in the blood– ocular barrier alteration27. Since Oxidative Stress is increased in the diabetic retina, the levels of oxidatively modified DNA and nitrosylated proteins are elevated, and antioxidant defense enzymes are impaired26. It has been reported that the level of antioxidant enzymes along with potential antioxidant vitamins are decreased in diabetic experimental animals and humans. Antioxidants may act at different levels, including the inhibition of the formation of reactive oxygen species (ROS), scavenging free radicals, or increasing the antioxidants defense enzyme capabilities.These functions are all related to the occurrence and development of diabetic complications. But in the past researches, uric acid was usually considered as a marker rather than a risk factor for the progression of disease. There is always a controversy that serum uric acid concentration is a cause or a result of microvascular complications27. Several lines of evidence suggest that increased plasma uric acid may be a significant risk factor of vascular disease. Ioachimescuet.al., believes that uric acid may causally/mechanistically contribute to vascular disease29. In our current study, the mean cholesterol, triglyceride, HDL and LDL cholesterol levels were higher in Group III compared to Group I & Group II. Our findings were consistent with the study done by Remaet.al,however, only triglycerides were independently associated with DR30. There are some conflicting reports in the literature regarding the effect of lipid profile on retinopathy. Chew et al., stated that patients with high Total Cholesterol and LDL levels were more likely to have retinal hard exudates compared to patients with normal lipid profile31. Moreover, patients with elevated serum total cholesterol, LDL-c, or triglyceride levels that did not have retinal hard exudate initially, were at increased risk of developing retinal hard exudate during follow-up. According to Idiculla J et al., and Sachdev N et al., retinal exudates or macular edema (ME) were associated either with higher levels of LDL or total cholesterol, or both32,33. Benarous Ret.al. reported that lipid profile was not associated with retinal thickness, mild or moderate Diabetic Macular Edema (DME) but only clinically significant with ME34. In the present study uric acid shows positive correlation with BMI, TC, HDL and LDL in Group III. BMI above 25 kg/m2 was one of the main component associated with UA increase, where BMI showed a positive relation with leptin concentrations, which a factor is leading to UA increase. Ultimately, obesity and DR may also be connected owing to increased oxidative stress as a result of its association with hyperleptinemia35. It was speculated that higher uric acid concentrations were associated with higher values for body adiposity markers (weight, BMI, WHR and OBI). Additionally, individuals with high BMI may show insulin resistance, TG alteration and high blood pressure, and all these factors are related to UA increase35. UA increase is observed in individuals with insulin resistance, probably because hyperinsulinemia would cause lower renal UA excretion35. Besides, insulin could also indirectly affect UA, since there is an association between hyperinsulinemia and hypertriglyceridemia. Studies conducted by Valtuena S et.al., reported that serum uric acid levels depends on insulin resistance and independent of age, sex, excess body weight, fat distribution and blood pressure36. Serum lipids may have a strong influence only in the severe forms of diabetic microvascular disease. They may not cause direct injury to the endothelium but are rather involved in the pathogenesis of DME only exudation of lipids through damaged retinal vasculature, which occurs at a later stage. Thus, it was suggested that serum lipids are involved in the later, more severe stages than in earlier stages; as an explanation to the discrepancies among the findings of the studies29. The limitations of our study included- gender variation, hospital based study instead of community based, population with T2DM, Some dietary factors were not evaluated, such as the intake of alcohol, purine and caffeinated drinks, which are known for their interference with UA values35.Since there may be dietary variations that were not analyzed, large multi-centric prospective studies are needed about this subject, especially to clarify the reasons of discrepancies between the findings of studies.
Conclusion
DR is a complex disease with several proven and some insufficiently verified proposed risk factors including inflammation. Hyperglycemia and oxidative stress in Type 2 DM leads to micro and macrovascular complications. We have shown that In Group III, there was positive correlation of serum Uric Acid with Anthropometric & Biochemical Indices. Dyslipidemia appears to be associated with the progression of DR in type 2 diabetes. Accordingly, it may be a relevant factor in a cascade involving the occurrence and development of DR. Our findings confirm the results of most of the previous investigations and illustrate the value of obesity assessment as an important modifiable risk factor which may consequently have potential clinical implications in the management of DR. Since weight gain is changeable and may be managed by lifestyle intervention. Therefore uric acid can be considered as a reliable marker which is less expensive and helps clinicians in controlling the progression of DM to microvascular complications like DR.
Acknowledgement
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res ClinPract2011;94:311-321.
2. Harris MI, Klein R, Welborn TA, Knuiman MW. The onset of NIDDM occurs at least 4-7 years before its clinical diagnosis. Diabetes Care 1992; 15:815-819.
3. Klein, BE, Klein. K, Moss. SE, “The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years,” Archives of Ophthalmology, 1984;102(4):520–526
4. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defence mechanism in humans against oxidants and radicals which cause aging and cancer: a hypothesis. ProcNatlAcadSci USA 1981;78:6858- 6862.
5. Nakagawa T, Zharikov S, Tuttle KR, Short RA, Glushakova O. A causal role of uric acid in the fructose- induced metabolic syndrome. Am. J. Physiol. Renal. Physiol. 2005;290:625-631.
6. Murea M Advanced kidney failure and hyperuricemia. Adv Chronic Kidney Dis. 2012;19:419-424.
7. Tassone EJ, Presta I, Sciacqua A, Rotundo M. Uric acid promotes endothelial dysfunction: A new molecular model of insulin resistance. Eur J Clin Invest 2011;41: 81-82.
8. P. Cirillo, W. Sato, S. Reungjui. “Uric acid, the metabolic syndrome, and renal disease, Journal of the American Society of Nephrology 2006;17(3):S165–S168.
9. Anwar MM,Meki AM. “Oxidative stress in streptozotocininduced diabetic rats: effects of garlic oil and melatonin,” Comparative Biochemistry and Physiology A2003;135( 4):539–547.
10. Hsu SP, Pai MF, Peng YS, Chiang C, Ho T. “Serum uric acid levels show a “J-shaped” association with allcause mortality in haemodialysis patients,” Nephrology Dialysis Transplantation 2004;19(2):457–462.
11. David B Sacks MB. Estimation of Blood Glucose. In: Teitz, Burtis CA, eds. Clinical chemistry and Molecular Diagnostics. 4thed.New Delhi: Elsevier, 1999, pp 870-871.
12. Edmund Lamb, David J, Cristopher P. Creatinine estimation. In:Teitz, Burtis CA, eds. Clinical chemistry and Molecular Diagnostics. 4th ed. New Delhi: Elsevier, 1999, pp 798.
13. Nader Rifai, G. Russell Warnick. Friedewald Equation. In: Teitz, Burtis CA, eds. Clinical chemistry and Molecular Diagnostics. 4thed. New Delhi: Elsevier, 1999, pp 842-843.
14. Levy AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 1990; 130:461- 470.
15. Roglic G, Unwin N, Bennett PH. “The burden of mortality attributable to diabetes: realistic estimates for the year 2000,” Diabetes Care 2005;28(9):2130–2135.
16. Flack JM, Peters R, Shafi T, Alrefai H, Nasser SA. “Prevention of hypertension and its complications: theoretical basis and guidelines for treatment,” Journal of the American Society of Nephrology 2003;14(2):S92–S98.
17. Kureja S, Malhotra N, Chhabra N. Correlation of the Serum Insulin and the Serum Uric Acid Levels with the Glycated Haemoglobin Levels in the Patients of Type 2 Diabetes Mellitus. Journal of Clinical and Diagnostic Research. 2013;7(7):1295-1297.
18. Oliveira E P, MoretoF, Arruda L V, Burini R C. Dietary, anthropometric, and biochemical determinants of uric acid in free-living adults Nutrition Journal 2013; 12:1-10.
19. Matthews D. R, Stratton I. M, Aldington S. J, Holman R. R, Kohner E. M, “Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69,” Archives of Ophthalmology 2004;122(11):1631–1640
. 20. Schrier RW, Estacio RO,Mehler PS. “Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: a summary of the ABCD trial,” Nature Clinical Practice Nephrology 2007;3(8):428– 438.
21. Srinivasa NR, Gurumurthy P, Gururajan P, SarasaBarathi A, Krithivasan V, Saibabu R, et al. Comparison between Serum Insulin levels and its Resistance with Biochemical, Clinical and Anthropometric Parameters in South Indian Children and Adolescents. Indian Journal Clin. Biochem. 2011; 26(1): 22-27.
22. Pagano G, Pacini G, Musso G Non alcoholicsteatohepatitis, insulin resistance and metabolic syndrome: Hepatology. 2002; 35: 367-72
. 23. Correa Z, FreiatasAM, Macron IM. Risk factors related to the severity of diabetic retinopathy. Arq Bras Oftalmol. 2003;66:739-743
. 24. Ishrat K, Jaweed SA, Bardapurkar JS, Patil VP. Study of magnesium,glycosylated hemoglobin and lipid profile in diabetic retinopahy. Indian J ClinBiochem. 2004;19:124- 127.
25. Butturini U, Coscelli C, Zavaroni I. Insulin release in hyperuricemic patients. Harefuah. 1995;128:681-683.
26. Terrasa AM, Guajardo MH, Marra CA, Zapata G Alpha-Tocopherol protects against oxidative damage to lipids of the rod outer segments of the equine retina. 2009; 182: 463- 468.
27. Kowluru RA Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal 2005;7: 1581-1587.
28. Plagemann PG. Transport and metabolism of adenosine in human erythrocytes: effect of transport inhibitors and regulation by phosphate, Journal of Cellular Physiology 1986;128,(3):491–500.
29. Ioachimescu AG, Hoogwerf BJ Comments on the letter by Pitocco et al. (Serumuric acid, mortality and glucose control in patients with type 2 diabetes mellitus: a PreCIS database study) Diabetic Medicine 2008;25(4):509.
30. Rema M, Srivastava BK, Anitha B, Deepa R , Mohan V. Association of serum lipids with diabetic retinopathy in urban South Indians-the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-2. 2006;23(9):1029-1036.
31. Chew EY, Klein ML, Ferris FL III, Remaley NA, Murphy RP, Chantry K,Hoogwerf BJ, Miller D. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22 Arch Opthalmol. 1996;114 (9):1079- 1084.
32. Sachdev N, Sahni A. Association of systemic risk factors with the severity of retinal hard exudates in a north Indian population with type 2 diabetes. 2010;56(1):3-6.
33. Idiculla J, Nithyanandam S, Joseph M, Mohan VA, Vasu U, SadiqM.Serum lipids and diabetic retinopathy: A cross-sectional study. 2012:16(Suppl 2):S492-494.
34. Benarous R, Sasongko MB, Qureshi S, Fenwick E, Dirani M, Wong TY, Lamoureux EL. Differential association of serum lipids with diabetic retinopathy and diabetic macular edema. Invest opthalmolvis sci. 2011; 52(10):7464-7469.
35. Valtuena S, Numeroso F, Ardigo D, Pedrazzoni M, Franzini L, Piatti PM, Monti L, Zavaroni I: Relationship between leptin, insulin, body composition and liver steatosis in non-diabetic moderate drinkers with normal transaminase levels. Eur J Endocrinol 2005; 153:283–290.
36. Larsson LI, Alm A, Lithner F, Dahlén G, Bergstrm R. The association of hyperlipidemia with retinopathy in diabetic patients aged 15-50 years in the county of Ume? ActaOpthalmolScand 1999;77(5):585-591.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License