IJCRR - 7(5), March, 2015
Pages: 56-62
Print Article
Download XML Download PDF
A CONCISE ENTOMOLOGICAL EVALUATION OF ONCHOCERCIASIS TRANSMISSION IN AHANI-ACHI COMMUNITY IN OJI-RIVER LOCAL GOVERNMENT AREA OF ENUGU STATE, NIGERIA
Author: Chikezie F.M., Ezihe E.K., Uzoigwe N.R., Opara K.N., Igbe M.A., Okonkwo N.J., Nwankwo E.N., Nwoke B.E.B.
Category: Healthcare
Abstract:Background and objectives: Onchocerciasis is an important threat to public health in Nigeria, which in turn contributes significantly to total world cases of the disease. This study determined the rate of transmission of onchocerciasis in Ahani-Achi, identified the principal vector groups of the disease in the study area and evaluated the relative abundance of black fly vectors and the various transmission indices. Methodology: Black flies were caught using human baits and were assessed for parity. Parous flies were further dissected to detect the presence of Onchocerca larvae. Biting rates and transmission potentials were calculated using standard methods. Results: A total of 836 adult female flies were caught in the community. These were identified as members of the forest species of the S. damnosum complex. The differences in relative abundance between the months were not significantly different (P > 0.05). The monthly biting rate (MBR) was lowest in February but highest in October. There was no on-going transmission in the area studied as no infective fly was caught. Hourly variation in fly activities were observed and this was significantly different (P< 0.01). Conclusions: The findings of this study signify that the forest black fly species are the major vectors of onchocerciasis in the area. Transmission in the study area is reduced but the presence of some larval stages of the filarial parasite in some flies signifies the possibility of transmission.
Keywords: Onchocerciasis, Black flies, Onchocerca, Vectors
Full Text:
INTRODUCTION
Onchocerciasis is a tropical parasitic filarial disease posing serious public health challenges. It negatively affects socio-economic development especially in Africa[1], and other negative impacts on areas that are endemic. The clinical manifestations of the disease include total loss of vision, partial visual impairment, skin lesions, hanging groin, and hernia [2], among others. Results of recent epidemiological studies revealed that 37 million people are infected with onchocerciasis, and 90 million at risk in Africa [3]. Nigeria is one country with more people blinded by onchocerciasis compared to any other country of the world [4]. It was estimated that 100,000 cases of the 268,000 worldwide cases, as well as approximately 3.2 million infections with O. volvulus occur in Nigeria [5]. Nigeria therefore accounts for more than one third of the total global onchocerciasis infection [6]. The genus Simulium comprises many species and complexes. In West Africa, the most dorminant of such complexes is the S. damnosum complex, comprising S. dam nosum s.s., S. sirbanum, S. sanctipauli, S. soubrense, S. yahense, S. squamosum, S. leonense, S. konkourense and S. dieguerense [7] [8] [9] [10]. In Nigeria, 9 cytoforms of the Simulium damnosum complex have been reported from different parts of the country. These include: S. damnosum s.s, S. sirbanum, S. sudanense, S. squamosum Volta form [11], S. squamosum Enderlein, S. yahense, S. sanctipauli, S. soubrense and the Beffa form of S. soubrense [12]. [13] found the main vectors species in Nigeria to be S. damnosum s.s, S. sirbanum, S. sanctipauli, S. soubrense S. squamosum, S. yahense. However, a cytotaxonomic analysis of Simulium damnosum s.1 larvae collected from 23 sites across 4 bioclimatic zones in Nigeria carried out by [14] revealed the presence of 5 cytospecies namely: S. damnosum s.str., S. sirbanum, S. squamosum, S. yahense and S. soubrense (including the Beffa form); excluding S. sanctipauli as one of the species found. Assessment of potential onchocerciasis vectors and their infection levels through capture and dissection of adult flies is an advantageous and noninsidious means for assessing the need for and success of various control measures [15]. It does therefore serve the purpose of monitoring levels and magnitudes of parasite transmission [16]. Fly infectivity rates vary with location. It is recognized that different cytospecies of the S. damnosum complex living in different biotypes may carry different quantities and strains of Onchocerca volvulus [17] [18]. The Nigerian Federal Ministry of Health through the National Onchocerciasis Control Programme (NOCP) have continued the assessment of onchocerciasis burden and frequency. Elimination of the disease as a public health problem via annual distribution of ivermectin to communities with high incidences was the sole purpose for establishing the NOCP. Its advent has remained a significant stimulus to research into the epidemiology and transmission of onchocerciasis in Nigeria [19] [20] [21] [22] [23] [24] [25] [16]. Onchocerciasis is indeed a disease of serious public health significance in Nigeria. A recent and comprehensive data of all communities with ongoing onchocerciasis transmission in Enugu State, and the nation at large is not available. However it is necessary for monitoring the effectiveness of control efforts and successes especially that by the NOCP. The objective of this study therefore is to update this information and to supplement this paucity of data with entomological observations made during the study period. This study therefore was designed to determine the role of vector black flies in the transmission of onchocerciasis in Ahani-Achi, identify the principal vectors groups of the disease in the study area and to evaluate the relative abundance of black fly vectors and the various transmission indices.
MATERIALS AND METHOD
Study area
The study was conducted in Ahani community (Lat 060 37’ N, Long 070 52’ E) of Achi town, Oji-River Local Government Area of Enugu State, Nigeria. This is an onchocerciasis meso-endemic community located along the Oji River basin [26]. It has an estimated population of 5,000 people according to the 2006 national census and comprises mainly of farmers, civil servants and petty business men and women. The community has two health facilities which include a Primary Health Care center and a private clinic. There are two seasons in the community namely: wet season (April to November) and dry season (December to March). The annual mean rainfall ranges between 1520-2030mm and the mean monthly temperature varies between 22.40 C and 30.8°C [27], characteristic of a tropical rainforest area. Oji River is a relatively large river, covered by dense forest and supplied with very minimal sunlight particularly so around the collection sites for this study. The study area is approximately 45 km from Enugu, capital of Enugu State and it is a semi-urban settlement. All adult fly samples collected for the purpose of this study were taken to the laboratory for identification and dissection. At about one week before the commencement of the study, an advocacy visit was made to the study community, and the health facility in the community. This avenue was used to sensitize these stakeholders. For reasons of assessibility of the breeding sites during the main rains, the study was carried out during the late rains of October-November 2011 (representing the rainy season), and January-February, 2012 (representing dry season).
Collection of man-biting adult black flies
Adult flies were collected along the banks of the Oji River basin at the designated community. Collection tubes were used for this purpose. Two consented and trained fly collectors were used for the human landing collection of adult black flies between 6:00 and 18:00 hours GMT. Transmission parameters were determined from data generated from the fly catches. The collectors were dressed in knickers or trousers folded to the knee level to expose the legs. They were very vigilant enough as to see and capture the black flies before they could bloodfeed.
Morphological identification
Morphological identifications of adult female flies were carried out in a small field laboratory using dissecting microscope, and according to the morphological identification keys of [28]. Adult black flies were identified morphologically as savanna or forest species on the basis of the colour of the 9th abdominal tergite setae, antennae, fore-coxae, scutella setae, wing arculus, and wing tufts. Those with pale wing tuft and pale procoxa were considered as savanna flies, those with pale or dark wing tuft and dark procoxa were considered as forest flies. Simulium damnosum s.s and S. sirbanum are considered savanna while other members of the complex were considered forest species.
Dissection of adult flies
The flies collected were dissected after having been identified as savanna or forest species. The dissection was carried out under a drop of physiological saline to prevent drying up and for easy visibility of the internal organs. All adult female flies collected were anaesthetized with chloroform to immobilize them before dissection. The flies were placed dorso-ventrally on a microscope slide containing a drop of physiological saline and dissection was carried out beginning from the posterio-ventral end of the abdomen to assess the ovaries and other internal organs for parity testing. Flies were recorded as parous or nulliparous indicating that they had, or had not taken at least one blood meal and had, or had not completed at least one gonotrophic cycle. Nulliparous flies had smaller and more compact ovaries, tightly coiled ovary tracheal systems, absence of follicular relics, and absence of retained eggs as well as dark and unbroken malpighian tubules. Parous flies had larger and more flaccid ovaries which are less elastic, loosely stretched ovary tracheal system, follicular relics are present below the maturing oocytes. They also had broken malpighian tubules which progressively have the appearance of a pale colour, and may contain retained eggs [29] [30]. All parous flies were further dissected minutely to detect the presence of O. volvulus larvae. The number of sausage-shaped larvae (L1 ), pre-infective (L2 ) and infective (L3 ) of Onchocerca species found in the abdomen, thorax and head, respectively were counted and their stages of development at these sites recorded.
Entomological indices
The fly density and level of transmission of onchocerciasis were quantified using two entomological indices, the monthly biting rates and transmission potentials. The monthly biting rates (MBR) were measured as the theoretical black fly bites received by a person stationed at a catching site during the twelve hours of the daylight for one complete month in a given community. The monthly transmission potential (MTP) was established as the total number of infective larvae (third stage larvae found in these black flies) that would be received in one month by an individual stationed at a capture point for 12 hours of the daylight.
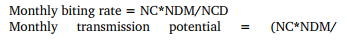
RESULTS
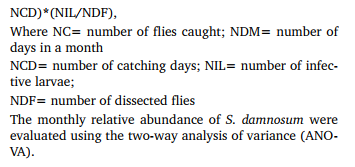
Relative abundance of adult female black flies A total of 836 adult female flies were collected in the study community during the entire study period. The highest number of flies (271) was caught during the month of October while February recorded the lowest number of 171 flies. A comparison of relative abundance of black flies caught during the entire study period showed that there was no significant difference between the monthly collections (P > 0.05). Table 1 shows the Summary of transmission indices of S. damnosum in the four collection months. In both rainy and dry seasons, the percentage monthly black fly parity rates remained high throughout the study. In each case, parity was above 50 %.
Diurnal biting rate
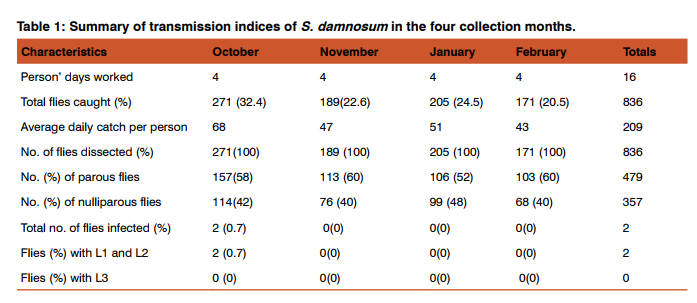
Hourly variations in diurnal pattern of fly biting activities were observed across the study period. In general, the fly biting activities showed a characteristic bimodal pattern, with morning and evening peaks. However, during unsteady weather conditions, there were other irregular and smaller peaks observed during the late mornings and early afternoons. The diurnal biting activities of S. damnosum in the study community is shown in Figure 1. The month of October had three peaks of biting activities at 09:00, 11:00 GMT and 16:00 GMT; November had two peaks at between 10:00 - 11:00 and 17:00 GMT; January had another three peaks at 10:00, 13:00 and 17:00 GMT. Fly biting activities for February peaked at 11:00 and 17:00 GMT. The lowest number of flies was collected during the hour of 7:00 while the highest was collected during the hours of 16:00 and 17:00 GMT. The diurnal biting activities of black flies between the months were significantly different from each other (P < 0.01). The total number caught by 6:00 GMT was significantly different from all other hours except 8:00 and 14:00 GMT. There were also differences between the other hours of collection.
Monthly biting rates
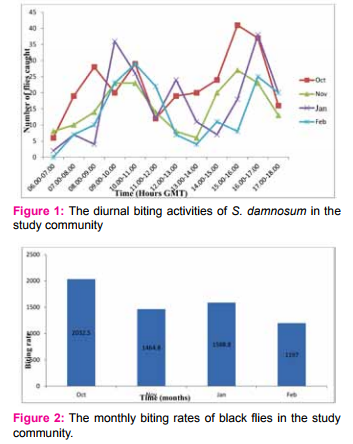
The monthly biting rates (MBR) of black flies in the study community were estimated for each month during
the study period as shown in Figure 2. Throughout this period, the month of October recorded the highest MBR of 2032.5 bites/person/month while the lowest MBR of 1197 bites/person/month was recorded in February. A comparison of the monthly biting rates showed that there was a significant difference between October and February MBRs (P < 0.01). October had a significantly higher MBR than February. However, there was no significant difference in biting rates between the other months using LSD mean separation technique.
Monthly Transmission Potential (MTP)
All the 836 flies collected for the purpose of this study were dissected to assess the O. volvulus infection rate. Out of the total flies dissected, only 2 flies were infected with first and second larval stages of O. volvulus, all in the month of October. One of these flies had 2 L1 larvae in the abdomen and 1 L2 larva in the thoracic region while the other only had 1 L2 larva in the thorax. These represent 0.7 % of the total catches for that month. No infective fly (flies with L3 larvae) was found throughout the studies (See table 1). The monthly transmission potentials for the four months were zero.
Morphological Identification of Adult Black
Flies The result of morphological identification showed that all flies collected were of the forest origin, characterized by dark colour of the 9th abdominal tergite setae, antennae, fore-coxae, scutella setae, wing arculus, and wing tufts. However, there were variations observed in the colour of the wing tuft. Some flies had pale wing tufts while the rest of the features were dark.
DISCUSSION
It is usually difficult to estimate the population of adult black flies, but this is needed for any meaningful vector control studies. Abundance indices are therefore generally based on density of females as determined from their landing and biting rates on man [31]. This is usually based on results of human landing catches. This method not only helps in determining population estimates but it is also a tool for monitoring disease transmission. Climatic and hydrological factors are known to affect adult black flies populations resulting in diurnal and sea sonal variations in fly populations [32] [33]. The density and distribution of black flies in the study area were found to be influenced by the presence and number of breeding sites, vegetation and other supports for larval and pupal attachment. There were also variations in the relative abundance of flies caught during different months of the study. The higher fly numbers observed during the month of October may be attributable to some physical parameters of the breeding sites such as water levels, pH values, water speed, availability of suitable rapids which created the necessary environment needed for development and survival of the aquatic stages of members of the S. damnosum complex, among other reasons. The observed relationship between biting rates and water levels of breeding sites in the study area were similar to those observed by [34]. As the dry season approached, there were observed reductions in water level and suitable rapids for breeding of vectors and these may have contributed to observed relative abundance of black flies especially in February. In this study, diurnal fly biting activities observed did agree with the bimodal biting activity reported by [35] and [20]. The month of January however showed a trimodal biting pattern and this is consistent with another report by [36]. These differences could be due to illumination factors, temperature, humidity or other climatic factors. Two findings, one from Guatemala and the other from Jos plateau in Nigeria agree that these factors indeed influence fly biting activities[37] [38]. Diurnal fly activities peaked in the morning between 9:00 and 10:00 GMT and in the evening between 15:00 and 17:00 GMT. These are periods of daylight for host seeking activities of black fly vectors in the community, coinciding with working habits of the people in the community, who are predominantly farmers, working usually close to the Oji River. They are therefore exposed to high risks of fly bites and infection from black flies. [39] and [40] observed a similar trend in fly activities. The minimum monthly biting rate (MBR) of (1197 bites/ person/month) and the maximum of (2032.5 bites/person/month) black flies observed in this study were all greater than the World Health Organization tolerable value of 1000 bites/person/month for ABR. This goes to indicate the high level of biting nuisance residents of Ahani-Achi community are enduring. Sustained high level nuisance could translate to onchocerciasis transmission should that opportunity present itself. No on-going parasite transmission was observed in this study in the community. There was absence of infective larvae in the head of local black fly vectors. Assessment of fly infectivity rates was used as a tool for determining the level of transmission in the area. It is reassuring and noteworthy that transmission was low as no infective fly was found during the study period. However, there were two cases of non-infective but infected flies. This suggests a possible interruption of onchocerciasis transmission, and yet heightens the need for sustained mass Ivermectin distribution in these areas to achieve local elimination of onchocerciasis. It also serves as a reminder for the necessity for more concerted research efforts to further monitor fly infectivity in the area due to the presence of L1 and L2 larvae in some flies. Such research studies may actually prove further the transmission status of Ahani-Achi community. The remarkably high parity rate of black flies found in this study may further be suggestive of the success of onchocerciasis control efforts. This is because one would expect high transmission in a place with high parity rate as these parous flies may be harboring the infective larval O. volvulus. It also shows that vector populations had access to blood meal, the source of which however wasn’t determined, and that they have high enough longevity to reproduce. Areas with high presence of migratory flies in line with the [5] report as well as that of [41] may as well show high parity rate. The WHO mass drug administration with Ivermectin has been a great boost to onchocerciasis control in Africa and the South and Central Americas [42]. For close to 25 years now, Ahani-Achi community has benefited immensely from this initiative through the Community Directed Treatment with Ivermectin (CDTI) [43]. As a good microfilaricide, the drug when administered clears approximately all the juvenile causative parasite. Ivermectin is administered annually in most parts of Nigeria where it is believed that adult female worms do not regain fully their maximum reproductive potential until about one year after treatment with ivermectin. With this treatment having lasted for close to two decades now in the study site, one will expect a significant decrease if not elimination of onchocerciasis in the area over time. There is a direct relationship between the presence and number of microfilariae on the subcutaneous tissues of the human host and parasite transmission by vector black flies. This is most likely an important factor that contributed to the low transmission status reported by the present study as vector black flies could not readily pick up microfilariae - infective stage of Onchocerca volvulus to the black flies, which eventually develop to the infective L3 stage to man. Onchocerca volvulus and Onchocerca ochengi can be co-endemic and both species cannot reliably be distinguished based on morphology alone. They are also vectored by the same black fly species. Interestingly however, no report of the co-endemicity of this two species has been documented in Ahani-Achi. The presence in this study of only members of the forest species of black flies is a good indicator of the strain of the parasite O. volvulus in that area and hence the form of onchocerciasis, recognizing strain differences in the parasite transmitted by local black fly species in West Africa. This, together with the forested nature of the study area, suggests that cutaneous (mild) onchocerciasis may be dominant in the area. Evidences were also found in some of the symptoms observed as well as reported during the course of this study. The presence of dark ninth abdominal tergite setae in all samples examined may be pointing towards S. yahense since the characteristics of the breeding sites here is not quite different from that known already for this species [14]. Again, the presence of flies with pale wing tuft may possibly be suggestive of S. squamosum as this species has been reported to occur in large rivers in the Eastern Nigeria [29], a region of Nigeria corresponding to the area of this study. Wing tufts however are somewhat less reliable feature for morphological identification (personal observation) because of variability tendencies from environmental effects. CONCLUSION The findings of this study signify that the forest black fly species are the major vectors of onchocerciasis in Ahani-Achi. Transmission in the area is reduced since the area had been mesoendemic to onchocerciasis [26] [44]. Presence however of non-infective larval stages of the causative parasite in some flies though not the infective stages, signifies the possibility of transmission. We have provided here a current baseline information on biting activities of the vectors in the area as well as the present nature of onchocerciasis transmission.
COMPETING INTEREST
The authors hereby declare that they do not have any competing interest associated with this paper
ACKNOWLEDGEMENT
The authors would like to thank all who contributed to the success of this work for their valuable comments and suggestions to improve the quality of the paper. Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Burnham, G. M. D. Onchocerciasis. The Lancet 1998, 351(9112): 1341–1346.
2. WHO. Onchocerciasis (River blindness), WHO information fact sheets 2002.
3. Basanez M-G, Pion SDS, Churcher TS. River blindness: a success story under threat? PLoS Medical journal 2006, 3: 1454–60.
4. Post RJ, Onyenwe E, Somiari SAE, Mafuyai HB, Crainey JL, Ubachukwu PO. A guide to the Simulium damnosum complex (Diptera: Simuliidae) in Nigeria, with a cytotaxonomic key for the identification of the sibling species. Annals of Tropical Medicine & Parasitology 2011, 105(4): 277–297.
5. Report of a WHO Expert Committee on Onchocerciasis Control. WHO Technical Report Series WHO, Geneva 1995, p 852.
6. WHO. Epidemiology of onchocerciasis. Report of WHO Expert Committee. Technical Report Series, No 752 Geneva 1987, 1 pp.
7. Dunbar RW, Vajime CG. The Simulium (Edwardsellum) damnosum complex. A report of cytotaxonomic studies to April 1972. WHO mimeographed document WHO/ONCHO/72.100 1972, p 10–23.
8. Vajime CG, Dunbar RW. Chromosomal identification of eight species of the subgenus Edwardsellum near and including Simulium (Edwardsellum) damnosum Theobald (Diptera: Simuliidae). Tropenmedizin and Parasitologie 1975, 26: 11–18.
9. Boakye DA. A pictorial guide to the chromosomal identification of members of the Simulium damnosum Theobald complex in West Africa with particular reference to the Onchocerciasis Control Programme area during the decade of 1984-93, following intensive larviciding since 1974. Medical and Veterinary Entomology 1993, 12: 345–358.
10. Boakye DA, Post RJ, Mosha FW, Surtees DP, Baker RHA. Cytotaxonomic revision of the Simulium sanctipauli subcomplex including the description of two new species within the Simulium damnosum complex in Guinea and surrounding countries. Bulletin of Entomological Research 1993, 83: 171–186.
11. Vajime CG, Gregory WG. Onchocerciasis: Species complex of vectors and epidemiology. Acta Leidensia 1990, 59: 235- 252.
12. Meredith SEO, Cheke RA, Garms R. Variation and distribution of forms of Simulium soubrense and S. sanctipauli in West Africa. Annals of Tropical Medicine and Parasitology 1983, 77: 627–640.
13. Nwoke BEB, Shiwaku K, Takahashi H. Nigeria onchocerciasis: Epidemiology perspective. The Nigerian Journal of Parasitology 1991, 22(1): 3–10.
14. Mafuyai HB, Post RJ, Vajime CG, Molyneux DH. Cytotaxonomic identification of the Simulium damnosum complex (Diptera: Simuliidae) from Nigeria. Trop Med Int Health 1996, 1: 779–785.
15. Ottesen EA, Ramachandran CP. Lymphatic filariasis infection and disease: control strategies. Parasitol Today 1995, 11: 129–131.
16. Opara KN, Fagbemi BO, Ekwe A, Okenu DMN. Status of forest onchocerciasis in the lower Cross River basin, Nigeria. Entomologic profile after five years of ivermectin intervention. Am J Trop Med Hyg 2005, 73(2): 371–376.
17. Philippon B. Etude de la Transmission d’Onchocerca volvulus (Leuckart, 1893) (Nematoda, Onchocercidae) oar Samarium damnosum Theobald (1903) (Diptera-Simulidae) Afrique Tropicale. Paris: L’Office de la RechercheScientifiqueet Technologiqued’Outre Mer. Travaux et Documents deL’ORSTOM 1977, 62–64.
18. Leveque C, Hougard JM, Resh V, Statzner B. Freshwater ecology and biodiversity in the tropics: what did we learn from 30 years of onchocerciasis control and the associatedbiomonitoring of west African rivers. Hydrobiologia 2003, 500: 23–49.
19. Okonkwo P, Akpa A, Ihekwaba A, Nwagbo D, Umeh R, Adibua S, et al. Studies on onchocerciasis in forest – Savannah Mosaic areas of Nigeria. Investigation in Gbaragu Oji River. Ann Trop Med Parasitol 1991, 38: 153–167.
20. Adewale B, Mafe MA, Oyerinde JO. Infectivity and transmission dynamics of Simulium damnosum s.l. around Owena Dam (Ondo State). West Afri J Med 1999, 18(4): 257–260.
21. Nwoke BEB, Dozie INS. Operational research and its success in onchocerciasis control in Nigeria. Nigeria Journal of Parasitology 2001, 22: 3–10.
22. Ubachukwu PO, Anya AO. Studies on the diurnal biting activity pattern of Simulium damnosum complex (Dipteran: Simuliidae) in Uzo-Uwani local government area of Enugu state, Nigeria. Nigerian Journal of Parasitology 2001, 22163–22168.
23. Oyibo WA, Fagbenro-Beyioko AF. Effect of repeated community-based ivermectin treatment on the intensity of onchocerciasis in Nigeria. Rural and Remote Health 2003, 3211–3216.
24. Idowu ET, Adewale B, Mafe MA, Appelt B, Bamgbose A. Endemicity of onchocerciasis in some local government areas of Niger state. African Journal of Medical Sciences 2004, 3331–3334.
25. Ubachukwu PO. Human onchocerciasis: epidemiological status of Uzo-Uwani local government area of Enugu state, Nigeria. Nigerian Journal of Parasitology 2004, 2593–2599.
26. Umeh RE, Chijioke CP, Okonkwo PO. Eye disease in an onchocerciasis-endemic area of the forest-savanna mosaic region of Nigeria. Bulletin of the World Health Organization 1996, 74 (1): 95–100.
27. State Ministry of Health Enugu. Demographic Profile of Enugu State Nigeria 2000.
28. Wilson MD, Post RJ, Gomulski LM. Multivirate morphotaxonomy in the identification of adult females of the Simulium damnosum Theobald complex (Diptera: Simuliidae) in the Onchocerciasis Control Programme area of West Africa. Annals of Tropical Medicine and Parasitology 1993, 87: 65–82.
29. WHO. Vector control series. Simulium. Advanced level training and information guide 1991, WHO/VBC/92.992. 1 pp.
30. Lewis DJ. Observations on Simulium damnosum Theobald at Lokoja in Northern Nigeria. Ann Trop Med Parasitol. 1958, 52(2): 216–231.
31. Crosskey RW. An appraisal of current knowledge of Simulium damnosum S. L. in the Federal Republic of Nigeria in relation to the development of an Onchocerciasis Control Campaign 1979, 1–11.
32. Crosskey RW Observations on the bionomics of adult Simulium damnosum (Theobald) (Diptera : Simuliiadae) in Northern Nigeria. Ann Trop Med Parasitol 1955, 49: 142– 153.
33. Crosskey RW The Natural History of Blackflies. Chichester, UK: John Wiley & Sons Ltd 1990, 5–26.
34. Le Berre R. Contribution à l’ étude biologique et écologique de Simulium damnosum Theobald, 1903 (Diptera Simulate) Memoires. O.R.S.T.O.M Paris 1966, 1pp.
35. Okolo CG, Akpa AU, Okonkwo PO. Studies on vectors of onchocerciasis and Simulium species in Achi, Oji River Local Government Area, Enugu State Nigeria. Nig J Ent 2004, 21: 84–9
36. Adeleke MA, Mafiana CF, Sam-Wobo SO, Olatunde GO, Ekpo UF, Akinwale OP, et al. biting behavior of Simulium damnosum complex and Onchocerca volvulus infection along the Osun River, Southwest Nigeria. Parasite and Vectors 2010, 3: 93–99.
37. Porter CH, Collins RC. Seasonality of adult black flies and Onchocerca volvulus transmission in Guatamala. Am J Trop Med Hyg 1988, 37: 153–167.
38. Nwoke BEB. Studies on the field epidemiology of human onchocerciasis on the Jos Plateau, Nigeria. The effects of climatic factors on the diurnal biting behaviour of Simulium damnosum (Theobald) (Diptera: Simuliiadae). Insect Sci Appl 1988, 91: 323–328.
39. Opara KN, Usip LP, Akpabio EE. Transmission dynamics of onchocerciasixs in rural communities of Akwa Ibom State, Nigeria. Journal of Vector Borne Disease 2008, 45: 225–230.
40. Renz A. Studies on the dynamics of transmission of onchocerciasis in a Sudan savanna area of North Cameroon III. Infection rate of the simulium vectors and Onchocerca volvulus transmission potentials. Ann Trop Med Parasitol 1987, 81: 239–252.
41. Henry MC, Meredith SEO. The onchocerciasis focus at Kinsuka/Kinshasa Republic of Zaire in 1985. Entomological aspects. Ann Trop Med Parasitol 1990, 84: 369–379.
42. WHO. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. World Health Organization. Geneva, 2010. Available from: http://www.who.int/neglected_ diseases/2010report/NTD_2010report_ embargoed.pdf
43. Onwujekwe EN, Shu EN, Nwagbo D, Akpala CO, Okonkwo PO. Willingness to pay for community-based ivermectin distribution: A study of three onchocerciasis endemic communities in Nigeria. Tropical Medicine and International Health 1998, 3(10): 802–808.
44. Nwaorgu OC, Ohaegbule A, Onweluzo IE, Alo ET, Nweke LN, Agu ML, et al. Results of a large scale onchocerciasis survey in Enugu State, Nigeria. J Helminthol. 1994, 68: 155–159.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License