IJCRR - 7(9), May, 2015
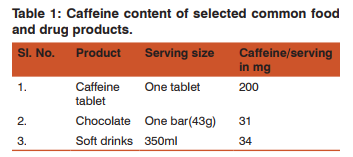
Pages: 16-19
Print Article
Download XML Download PDF
CAFFEINE EXTRACTION AND CHARACTERIZATION
Author: Pradeep S., G. N. Rameshaiah, Hadagali Ashoka
Category: General Sciences
Abstract:Caffeineextracted and characterised from tea (black) leaves and coffee beans. Isolation was done by liquid-liquid extraction using di-chloromethane as an extracting agent. This extraction was done in four steps: steeping, evaporation, liquid-liquid extraction and recrystallization. The recrystallization was done using anhydrous sodium sulphate.The technique used for purity analysis and characterisation were: High performance liquid chromatography, Differential scanning calorimeter, Fourier transform infrared spectroscopyand Melting point. First, the analysis was done using melting point analysis. The melting point of caffeine
extracted from coffee beans and tea leaves was found to be 238\?C. The absorption bands were compared with that available in literature and were found to be similar. Further, the purity check was done using High performance liquid chromatography method.Effective characterization of caffeine was achieved by determining Infrared spectrum, and employing a melting point apparatus and differential scanning calorimeter. The purity showed that the results that the extracted coffee was 90% pure. Further improvements in extraction efficiency will increase the yield and minimize wastage.
Keywords: Caffeine, Methyl xanthine, Theophylline, Differential scanning calorimeter, Fourier transform infrared spectroscopy, High performance liquid chromatography
Full Text:
INTRODUCTION
Caffeine is a psychoactive CNS stimulant drug discovered by German chemist Friedrich Ferdinand Runge in 1819. He coined the term ‘Kaffein’ which became Caffeine5 .Caffeine is a methyl xanthine along with theophylline and theobromine.It is a natural pesticide.Caffeine does not counteract the effects of alcohol. Caffeine is a xanthine alkaloid compound that acts as a stimulant in humans. It is a central nervous stimulant, having the effect of temporarily warding off drowsiness and restoring alertness.2 Every time we drink tea, coffee, cocoa, chocolate or cola we are giving our body a “hit” of caffeine. Along with nicotine and alcohol, caffeine is one of the three most widely used mood – affecting drugs in the world5 .
OBJECTIVES
- To extract caffeine from tea leaves and coffee beans by liquid-liquid extraction method.
- To characterize the obtained caffeine by melting point, Infrared spectroscopy and Differential scanning calorimetermethod.
- To develop an easily adaptable method for the qualitative or purity analysis of caffeine
PHYSICAL AND CHEMICAL PROPERTIES
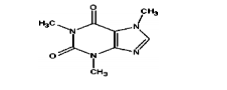
Caffeine is sparingly soluble in most polar solvents but is highly soluble in less polar solvents. The melting point is 234°C-239°C and the chemical formula is C8 H10N4 O2 .It is an intensely bitter, white powder in its pure state. Caffeine is an alkaloid of the methylxanthine family, which also includes the similar compounds theophylline and theobromine2 .The structure of caffeine is
ADMET OF CAFFEINE
Absorption and Distribution
Caffeine is absorbed orally with a max blood peak after 120 mins spreading quickly in all tissues.Caffeine is classified as a stimulant because it increases the activity of cardiovascular, digestive and sympathetic nervous system, and produces the sense of alertness in the brain. It can have a lethal effect ( acute intoxication) when ingested at amounts of 1-5 g, with plasma concentrations higher than 80mg/ml and the first intoxication signs appear at about 250mg4 .
Metabolism and Elimination
Hepatic metabolism becomes longer and more difficult in presence of alcohol and medical drugs while cigarette smoke accelerates its hepatic metabolism.Only 10% is eliminated through the kidney as unmodified caffeine4 .
Caffeine intoxication
The symptoms include restlessness, nervousness, and excitement, insomnia, flushing of the face, increased urination, gastro-intestinal disturbance, muscle twitching, and psychomotor agitation.The treatment is based on serum levels of caffeine which may be followed by peritoneal dialysis, hemodialysis, or hemo filtration. Caffeine stimulates acid production in the stomach so it is better not to drink coffee in case of gastric ulcer4
EXTRACTION OF CAFFEINE
Steeping procedure is used.The solvent used is dichloromethane.Purification methods like distillation and recrystallization are also followed.The success of extraction involving a natural product is often expressed as percentage recovery

The percentage recovery is called the purified percent recoveryor crude percent recovery. The extraction with the highest percent recovery is considered the most successful extraction5 .
MATERIALS REQUIRED
5g of tea leaves, 10g coffee beans, 4g of calcium carbonate, 2g sodium carbonate, 25ml methylene chloride, anhydrous sodium sulphate, Whatmann no.1 filter paper,15ml of dichloromethane,50ml and 500ml of Erlenmeyer flask, separating funnels, MilliQwater,etc.
CHARACTERIZATION OF CAFFEINE
Based on various physical methods.
1. Determination of melting point
2. Infrared spectroscopy
3. Degradation by Differential scanning calorimeter
4. Purity check by High performance liquid chromatography
RESULTS
Extraction of caffeine from tea and coffee was achieved by using chloroform as an extracting solvent
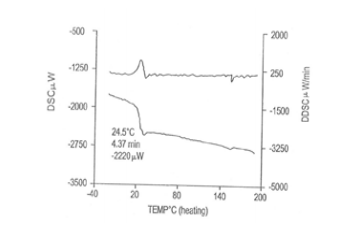
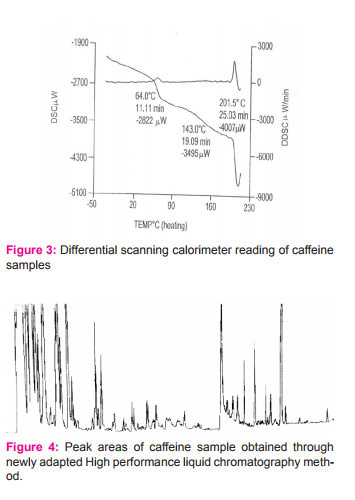
DISCUSSION
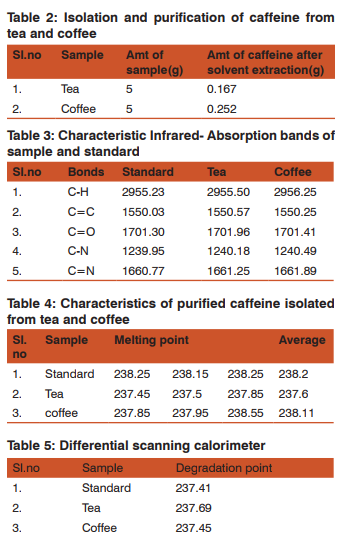
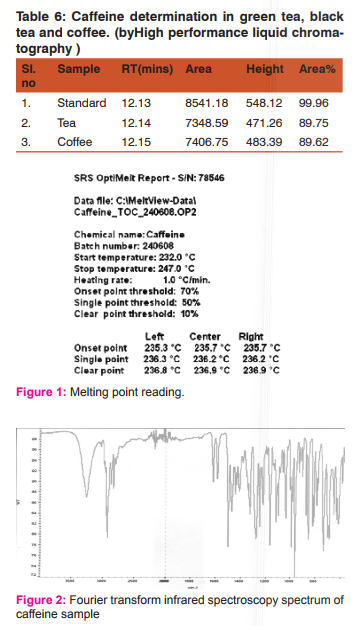
It was observed that the extraction efficiency of caffeine from various sources by using chloroform was much higher than other solvents. Table 2 shows the extraction efficiency of crude caffeine from tea and coffee leaves. The amount of caffeine obtained from L- L extraction after further recrystallization was found to be 3.37% from tea and 5.04% from coffee.We observed that coffee contained a high percentage of crude caffeine as compared to tea. To purify the crude caffeine, similar procedures were utilized. However, the caffeine content of various sources varies with soil conditions and climate. Table 3-6 shows the characteristics of caffeine in various parameters for both sample and standard respectively. Also 2-5 shows the result of caffeine samples being measured respectively. The pure white crystalline caffeine isolated from sources was found to melt at 238o C. The Infrared-spectrum of isolated caffeine showed similar absorption bands similar to that given in literature. The Infrared-spectrum indicates the absolute purity of the purified caffeine.
We have developed a High performance liquid chromatography method for the determination of caffeine, which was carried by High Performance Liquid Chromatography instead of using UV- Visible spectrophotometer. We chose High performance liquid chromatography method for the determination of caffeine, because High performance liquid chromatography is the most widely used qualitative and quantitative determination and separation method. This method is popular because it is non-destructive and unlike gas chromatography may be applied to thermally liable compounds. Moreover it is also a very sensitive technique as it incorporates a wide range of detection methods. With the use of post column derivatization methods to improve selectivity and detection limits, High performance liquid chromatography can easily be extended to trace determination of compounds that do not usually provide adequate detector response. The wide applicability of High performance liquid chromatography as a separation method makes it a valuable separation tool in many scientific fields. By using this method, we determined the retention time and the relative peak area of extracted purified caffeine. The retention time of the purified caffeine and that of the standard caffeine were almost similar, which confirmed the identity of caffeine. The amount of extracted purified caffeine for different samples was determined by studying the relative peak area on the calibration curve.
CONCLUSION
A method has been developed for the extraction, purification of caffeine from tea and coffee. Caffeine from tea and coffee was extracted by liquid- liquid extraction followed by recrystallization. The purified caffeine was then analysed by High performance liquid chromatography. Effective characterization of caffeine was achieved by determining IR spectrum, and employing a melting point apparatus and differential scanning calorimeter. The serious concern about potential use of caffeine for pathogenic effects has made it one of the most broadly studied drugs. It provides clinicians with the information they require in order to understand, diagnose and treat the effects of caffeine consumption in their patients.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. Authors are also grateful to authors, editors and publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Barone, J.J., Roberts, H.R. (1996) Caffeine Consumption , Food Chemistry and Toxicology, McGraw-Hill, Newyork,34, 119
2. Arnaud, M. J. (1987) The Pharmacology of Caffeine, Prog Drug, 31, 273.
3. S. Mathkar, S. Kumar, , A. Bystol, K. Olawoore, D. Min, R. Markovich, A. Rustum: The use of differential scanning calorimetry for the purity verification of pharmaceutical reference standards,Journal of Pharmaceutical and Biomedical Analysis, Volume 49, Issue 3, 5 April 2009, Pages 627–631.
4. H. T. Debas, M. M. Cohen, I. B. Holubitsky, and R. C.Harrison. Caffeine-Stimulated Acid and Pepsin Secretion: Dose-Response Studies, Scandinavian Journal of Gastroenterology, August 1971, Vol. 6, No.
5 , Pages 453-457. 5. Md. Abdul Mumin, Kazi Farida Akhter, Md. Zainal Abedin, Md. Zakir Hossain: Determination and Characterization of Caffeine in Tea, Coffee and Soft Drinks by Solid Phase Extraction and High Performance LiquidChromatography, Malaysian Journal of Chemistry, 2006, Vol. 8, No. 1, 045 – 051.
6. Islam, M. S, Rahman, M. M., Abedin, M.Z. (2002) Isolation of caffeine from commercially available available tea and tea waste, Jahangirnagar Uni. J. Sci.,25,9.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License