IJCRR - 7(9), May, 2015
Pages: 10-15
Print Article
Download XML Download PDF
DIVERSITY AND DISTRIBUTION OF AQUATIC INSECTS IN SOTHUPARAI RESERVOIR, AT PERIYAKULAM, THENI DISTRICT, TAMILNADU, INDIA
Author: Medona Mary R., Nirmala T., M. R. Delphine Rose
Category: General Sciences
Abstract:The present study deals with the diversity and distribution of aquatic insects from Sothuparai Reservoir. It is located at the foothills of the Western Ghats. The aquatic entamofauna were sampled systematically and randomly using standard protocols. The aquatic insects act as an indicator species to monitor the environmental pollution. Ephemeropteran were most diverse and its presence indicative of good water quality. The abundance of organic pollution tolerant Baetis were found in downstream, nearer to human settlements. The physico chemical variations of water taken into account for the study were found to be influencing
the distribution of aquatic insects. It is suggested that routine bio monitoring of the reservoir using aquatic insect indicators will facilitate better conservation and management.
Keywords: Aquatic insects, Diversity, Water quality, May flies, Sothuparai reservoir and Bio indicator
Full Text:
INTRODUCTION
India is one of the mega diverse countries, with a notable diversity of aquatic habitats of about 3,166,414 Km2 with significant variations in rainfall, altitude topography and latitude. The Western Ghats are, also called as Sahyadri hills, one of the tropical biodiversity hotspot in India. The diverse climatic conditions, physical and geographical nature of the Ghats renders shelter for rich fauna and flora. The Western Ghats is bestowed with a number of perennial and intermittent, small and large streams and rivers, the region shows high species diversity. The Ghats not only supports terrestrial but also aquatic diversity of flora and vertebrate fauna and least is the importance to invertebrate fauna ( Ramachandran et al., 2010). The linear morphology of streams and river is unique. The water current is one of the salient stream features but it varies seasonally, with depth and throughout the longitudinal profile of the water course. Freshwater habitats including both lentic and lotic habitats serve as a home to greater entamofauna. Among the 56% of insects, only 3-5% is aquatic and they are minor fraction of all insects. Nearly 3% of all the insects initiate their life cycle as aquatic larvae before emergence as winged terrestrial forms (Daly, 1996). The habitats for insect communities in streams show a great variation of aquatic invertebrate diversity. Cascade, riffles and pools are the most common stream habitat. Cascades are the habitats where the water flows turbulently through boulders and cobbles. Woody debris and litter get collected in the cascades because of its physical structure. The water flows with little turbulence over gravel and sand is the riffle. Pools are habitats with minimum water flow and least turbulence (Subramanian, 2003). Studies of invertebrate fauna of lentic ecosystems were correlated to species – habitat relationship with regard to the environmental variables (Compin and Cerghino, 2003; Azrina et al., 2005). Over 95% of the total individual in fresh water particularly streams comprise of these immature life stages of aquatic insects. They play an important role in food chain of stream ecosystem. Some freshwater insects have specific requirements regarding their nutrients, water quality, substrate and vegetation. The impact of human on freshwater were assessed by the use of indicator species (Carter and Resh, 2001; Nagendran, N., 2007). Indicator species are those taxa known to be par ticularly sensitive to specific environmental factors. Any changes in their incidence and abundance may directly reflect an environmental change (New, 1984). The aquatic insects, that have one of more life stages adapted for living in the aquatic environment, which may takes a few weeks to several years. Three aquatic orders, Ephemeroptera, Plecoptera and Odonata have a hemimetabolous life cycle. The other aquatic orders, Trichoptera and Megaloptera are holometabolous life cycle. Heteroptera has a paurometabolous life cycle. Among these orders Ephemeroptera, Plecoptera, Trichoptera, Coleoptera and Diptera are found in abundance in many streams (Subramanian, K.A., 2007 and Sivaramkrishnan, 2005). Most of the stream ecosystems are becoming increasingly polluted by domestic sewage, agricultural runoff, urban waste and industrial effluents (Trivedy and Goel, 1985; Trivedy, 1988, 1990; Kumar, 2001). Anthropogenic impacts on the structure and the organisms of freshwater ecosystems are diverse, unpredictable and unaccountable. It leads to structural and functional disturbance in freshwater ecosystem and finally reduces the biodiversity at different levels of biological organization. The use of living organisms to assess water quality is a century old approach to water quality evaluation. Physical and chemical data reflect a condition that exists during sampling. But biological monitoring gives an indication of past conditions also. The use of aquatic insects as bio indicators provides data to estimate the degree of environmental impact and its potential effects on other living organisms (Wahizatul, et al., 2011). In general, aquatic insects are largely ignored in the contemporary estimation on Indian biodiversity and hence the present study documents the diversity of aquatic entamofauna in Sothuparai reservoir, Theni District, Tamilnadu, India
MATERIAL AND METHODS
Description of Study site Sothuparai is located at 9 km from periyakulam and situated between the longitude 770 28’ 4’’ and latitude 100 7’ 45’’. Sothuparai dam supplies water to periyakulam throughout the year. Irrigation under sothuparai system 2,865 acre. Water spread area of maximum water level is 48.64 square meter. Maximum flood discharged allowed, 807.48. Full reservoir level is 405.5cm. Length of dam is 345 meter. Height of the dam is 1035.00 feet. Maximum water level is 100.22 feet. This stream is across the Varaha River.The wide expanse of stored water is an impressive sight.
Sample Collection
The water samples for the present study were collected once a month from the upstream and downstream of the reservoir between 10 am to 11 am. Sterlized sampling bottles were used to collect the water samples. After collection, the samples were kept in ice cold box before transporting to the laboratory
Analysis of water quality parameters
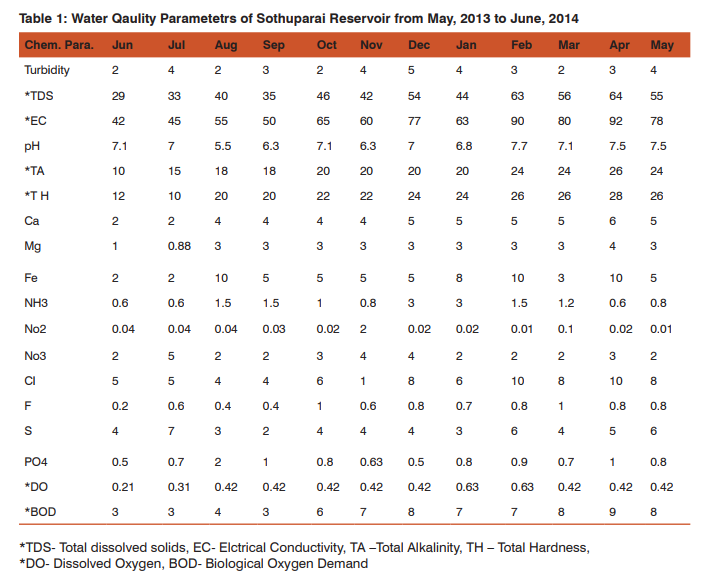
On the sampling spot, water quality parameters viz temperature, humidity, water current, depth and width of the stream and pH were measured. Width and depth of the streams were measured using a marked pole and measuring tape at each stream. Total Alkalinity, Total Hardness were analyzed by volumetric means. The chemical parameters such as Calcium, Iron, Magnesium, Nitrate, Nitrite, sulphate, Phosphate, DO, and BOD were analyzed in the laboratory following the standard methods as prescribed by APHA, (2005).
Sampling of Aquatic Insects
The study was conducted during the early hours of the day from June, 2013 to May, 2014. Two sites were selected for the study i.e. upperstream and downstream. A length of 100m reach was considered as a unit and the aquatic entamofauna were sampled using D- frame dip net as also kick net both of which are of 500µm mesh size. The Kick net was placed in the upperstream and downstream. One meter above stream bottom substrates was kicked to dislodge invertebrates clinging to debris and stones into the kick net. The contents were emptied into the tray and invertebrates were collected. The D frame net was employed to trap specimens clinging to vegetation, root mats etc., along the boundary (Merit and Cummins, 1988). Riffles and pools were sampled separately to account for sub habitat variations (Subramanian and Sivaramakrishnan, 2007). The collected specimens were preserved in jars containing 70% ethanol. They were identified using a LABOMED stereo zoom microscope with the help of standard keys (Merit and Cummins, 1988; Dudgeon, 1999 and Subramanian and Sivaramakrishnan, 2007).
Data Analysis
Species diversity indices such as Shanon -Weiner, Eveness were computed to understand the biotic community of each study site. Shanon –Weiner diversity (Shannon and Wiener, 1949) index helps in species relative abundance, evenness index is used for the degree to which the abundances are equal among the species present in a sample.
RESULTS
Samples Collected
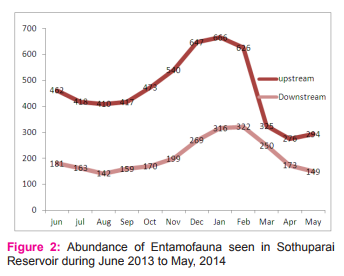
A total of 7243 individuals of entamofauna representing 43 genera categorized under 332 families and 9 orders were collected from the upstream and downstream of the Sothuparai Reservoir. The aquatic entamofauna of upper stream constituted 43 genera, 32 families and 9 orders, while in downstream it was recorded as 35 genera, 27 families and 9 orders. The abundance of entamofauna at Sothuparai Reservoir was recorded maximum in upper stream and minimum (2518) in downstream (Figure 2)
Composition of insect taxa in sothuparai Reservoir
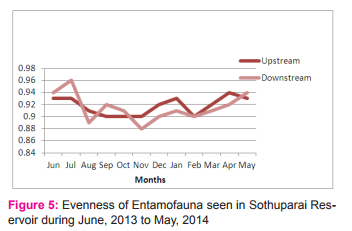
Figure 3 depicts the species diversity and percentage composition of various insect orders collected from the Sothuparai Reservoir. The highest numbers of taxa were in the order Ephemeroptera, while the Hemipterans had the highest number. Hemiptera showed the highest numerical abundance (36.73%) of the total insect fauna. It was represented by 8 families Viz., Hydrometridae, Belastomidae, Gerridae, Ranatridae, Notonectidae, Nepidae, Naucoridae and Corixidae. The diversity of the Aquatic entamofauna in upper stream is higher than in downstream. The evenness was high in upperstream whereas it was lower in down stream. In both the upper and downstream the evenness was so high during the month of July whereas it was low in December.
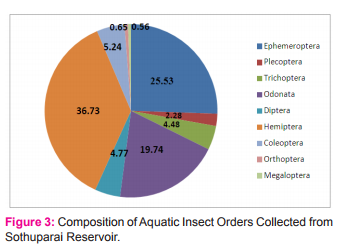
Out of the eight families Gerridae was the dominant family both in the upper stream and downstream of the Reservoir. This was followed by Hydrometridae, Notonectidae, Nepidae, Ranatridae, Belastomidae, Corixidae and Naucoridae.
DISCUSSION
Ephemeroptera
The Ephemeroptera is one of the intolerant group of insects which are consider as an indicator of water quality because of its presence in both the polluted and unpolluted reaches of the aquatic body.Thalerosphyrus belonging to the Heptagindae family was found to be abundant in upstream and absent in downstream. However, it appears to be intolerant to pollution (Abhijna et al., 2012). Tim, 1997; Menetrey et al., 2008 and Abhijna et al., 2012 reported that the genera Baetis species were tolerant top organic pollution. Arimoros and Muller (2010) stated that the overall composition and density of Ephemeroptera depends on the physico chemical and biological factors of the environment. The present study documents the Ephemeroptera taxa richness and diversity remain at a relatively high in the upper stream but reduced drastically in downstream of the reservoir.
Plecoptera Fore et al., (1996) stated that the order Plecoptera is highly sensitive to environmental degradation. In our present study the presences of stone flies were high in upper stream and very low in downstream. It was represented by only one genus Perlidae.
Trichoptera They were contributed by 6 genera Hydropsychidae, Lepidostomatidae, Helicopsychidae, Calamoceratidae,
Pilopotamidae and Psychomyidae. The numerical abundance of caddis flies was equal in both the streams of the reservoir. Sivaramakrishnan, et al (2000) concluded that Trichoptera as the most popular order of aquatic insects in the streams of Western Ghats in terms of the total abundance but it was contrary to our present findings of Sothuparai Reservoir in the Southern foot hills of Western Ghats.
Odonata
Odonata contributes 19 % of the total fauna. Libelluidae, Gomphidae and Euphaeidae were the families belonging to Odonata. The nymphs of this family remain attached to macrophytes. Crocothemis was the species of the Libelluidae, the naid of which is mud dwelling. 16 genera in Western Ghats have been collected by Subramanian and Sivaramakrishnan, (2005).
Diptera
The composition of dipterans in the Sothupari Reservoir was 4.76 % of the insect fauna. Tipulidae and simulidae are the families which belong to the order Diptera. Courtney, (2009) studied that dipteran species can be considered aquatic and they require a moist to wet environment within the tissues of living plants, decaying organic materials as parasites or in association with bodies of water.
Orthoptera , Megaoptera and Coleoptera
Orthoptera and Megaloptera were least in number of the total insect fauna recorded in Sothuparai Reservoir. Coleopterans were 5. 27 % of the insect communities in the Reservoir. It was represented by 6 genera categorized under 4 families. Dineutus was high in number abundance in upper stream, where as in downstream Gyrinus species was high and both of them belongs to Gyrinidae family. The major family of aquatic Coleoptera was Gyrinidae, Hydrophyllidae, Psephenidae and Noteridae. Khan and Gosh, 2001 observed that the Hydrophylidae family was water scavenger beetles and they were present in shallower regions of wetland. The emergent ones of Hydrophylide feed mainly on detritus, Algae and decaying vegetative matter. Nagendran, (2004) reported 7 families in selected streams in the three states of Southern Western Ghats.
Diversity and Evenness
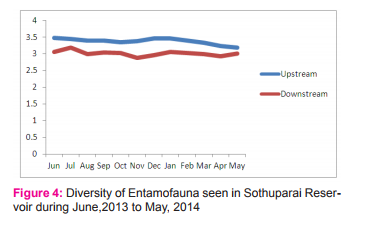
Diversity of aquatic entamofauna was high during June and it was low in May whereas, in downstream the diversity was high in June and low in November (Figure 4). This is in par with Dinakaran and Anbalagan (2007) and Kalayarsi (2008).
The present study reveals that the evenness value was recorded high in downstream (0.96) and in upperstream it was slightly low (0.94) indicating relatively more or less even distribution of species in the reservoir (Figure 5).
Water Quality Parameters The water quality parameters of the Reservoir were within the Permissible Limit as described APHA, (2005) (Table 1) (Sumitha and Rupali, 2013).
EPT Taxa Richness
The percentage of EPT index indicates the richness in upper stream and 25.80 % in downstream and this is in par with the results observed by Abijna et al., (2012) and Wahizatul et al., (2013). Thus the results indicated a better water quality was recorded in upper streams compared with the downstream of the Reservoir.
Anthropogenic activities might change the normal development of these fragile ecosystems especially at downstream of the Reservoir. This may be because of the several human activities such as recreation; agricultural activities and this in turn affect the diversity of aquatic insect communities. The present study clearly documents that the composition of the aquatic insect communities is moulded by their different levels of sensitivity to pollution in accordance with the abiotic factors in the stream ecosystem.
CONCLUSION
The physico chemical parameters and the aquatic insect communities together indicated the natural and manmade influences. Thus the study states that the biomonitoring of the Reservoir using benthic macro invertebrates in running water is the effective tool for the better management of the Sothuparai Reservoir.
ACKNOWLEDGEMENT
Authors express their deep sense of gratitude to University Grants Commission and Jayaraj Annapackiam College for Women (Autonomous), Periyakulam for providing the fund and the laboratory facilities. Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Abhijna, U G, Ratheesh, R, Bijukumar A, 2013. Distribution and Diversity of aquatic insects of Vellayani Lake in Kerala. Journal of Environmental Biology, 34: 605-611.
2. Arimoro, F O, W J, Muller., 2010: May Fly (Insecta: Ephemeroptera) Community structure as an indicator of the ecological status of a stream in the Niger Delta area of Nigeria. Environmental Monitoring assess, 166: 581-594.
3. APHA: Standard Methods of Examination of Water and Waste water.21st Edn. APHA, AWWA And WPCF publications, Washington Dc, USA.
4. Arienzo, M, Adamo, P, Bianco, M, Violanta, P, 2001. Impact of land use and urban runoff on the contamination of the Sarno river Baasin in Southern Italy. Water, Air, Soil Pollution. 131- 349, 366.
5. Armiatge, P D, Moss, D, Wright, J F, Fruse, M T, 1983. The performance of a new biological water quality score system based on macroinvertebrattes over a wide range of unpolluted running water sites. Water Research, 17: 333-347.
6. Azrina, M Z, Yap, C K, Rahim Ismail, A, Ismail, A , Tan, S G, 2005. Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Langat River, Peninsular Malaysia, Ecotoxicology and Environmental Safety, 16: 184-210.
7. Balchandran, C, Chandan, M D S, Ramachandran, T V, 2011. Distribution and Biology of the Western Ghats, Sahyadri eNews, 35.
8. Carter, J L, Resh V H, 2001: After site selection and before data analysis sampling, sorting and laboratory procedures used in stream using benthic macroinvertebrates monitoring programme by USA. J.N. Am. Benthol. Soc., 20: 658 -682.
9. Compin, A, Cereghino, R, 2003. Sensitivity of aquatic insect species Richness to disturbance in the Adour – Garonne stream system (France). Ecological Indicators. 2: 345- 360.
10. Courtney, G W, Pape, T J, SkeVington, H, Sindair, B J, 2009. Biodiversity of Diptera. In: Insect Biodiversity: Science and Society. (Edn: R. Footit and P. Adler) I1t Edn: Blackwell Publishing LTd, Oxford, UK. 185- 222.
11. Daly, H V, 1996. General classified key to the orders of aquatic and semi aquatic insects In: An introduction to the aquatic insects of North America (Eds. Merrit R.W.and Cummins, K.W) Kendall Hunt, Iowa, USA. 101-112.
12. Dudgeon, D, 1999. In tropical Asian streams – Zoorenthos, Ecology and Consolation, Hong Kong University Press, Hong Kong. 828.
13. Fore, L S, Karr, J R, Wisseman, RW, 1996. Assessing invertebrate response to human activities: evaluating alternative approaches. Journal of North America Benthol. Soc., 15: 212-231.
14. Kumar, A, 2001. (Ed.), Ecology of Polluteed waters, Vol.I and II. Ashish Publishing House, New Delhi. 1233.
15. Menetrey, N, Oertli, B, Sartori, M, Wagner, A, Lacha, J.B, 2008.Vanne: Eutrophication: Are May flies (Ephemeropteera) good bioindicators for ponds. Hydrobiolgia, 579:123-135.
16. Merrit, R W, and Cummins, K W, 1988. An Introduction to the aquatic insects of North America (2nd Edn.) Kendall. Hunt Publication Company, Dubuque, Iowa: 722.
17. Nagendran, N A, 2007. Stream Characteristics and biomonitoring of Perumal Patha Odai, Karanthamalai, TamilNadu. Ecol. Env and Cons.13 (1): 57—62.
18. New, T R, 1984. Insect Conservation Dr. W. Junk Publishers, Dordrecht, Netherlands.188-198.
19. Ramachandra, T V, Chandran , M D S, Bhat, H R, Dudani, S, Rao, G R, Boominathan, M, Mukri, V, Bharath S, 2010. Biodiversity, Ecology and socio economic aspects of Gundia river basin in the context of proposed mega hydroelectric power project, CES Technical Report -122, Centre for Ecological Sciences, Indian Institute of Science, Bangalore
. 20. Shannon, C E, Weiner, W, 1949. The Mathematical theory of communication. University of Illinosis press, Urbana: 117.
21. Subramanian, K A, 2003. Stream insect communities of Western Ghats and their bioindicators potentials (Thesis submitted to Madurai Kamarj University, Tamil Nadu)
22. Subramanian, K A, Sivaramakrishnan, K G, 2007. Aquatic insects of India: A field guide.
23. Ashoka Trust for Research in Ecology and Environment (AITREE), Bangalore.
24. Susmita Gupta, Rupail Narzary, 2013. Aquatic Insect community of lake, Phulbari Anua in Cachar, Assam. Journal of Environmental Biology, 34: 591-597.
25. Timm, H, 1997: Ephemeroptera and Plecoptera larvae as environmental indicators in running waters of Estonia.
26. Trivedy, R K and Goel, P K, 1985.(Eds). Current Pollution Researches in India. Environmedia. Karad, M.P: 344.
27. Wahizatul, A A , Luna, S H, Ahamed, A, 2011. Composition and distribution of aquatic insect communities in relation to water quality in two freshwater streams of Hulu Terengganu, Terengganu. Journal of Sustainability Science and Management, 6, (1).
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License