IJCRR - 7(12), June, 2015
Pages: 36-43
Print Article
Download XML Download PDF
DELAYED DIABETIC WOUND HEALING: A FOCUS ON BACTERIAL PROTEASES IN CHRONIC WOUND AND FOOT ULCER
Author: Saumya Mary Mathew, Varshaniyah Ravisanker, Tanvi Potluri, Suchithra T.V.
Category: Healthcare
Abstract:Background: The infectious bacteria produce proteolytic enzymes which help them to invade, establish infection and to survive the host defence mechanism delaying the wound healing. Objectives: Protease secreting potential of bacterial flora; specifically the bacterial isolates of diabetic ulcer foot patients are studied here. Methods: The predominant bacteria in foot ulcer were identified and bacterial enzymes caseinase, gelatinase, alkaline protease, hyaluronidase, proteinase K and collagenase were analysed. Results: Out of the 78 strains isolated S.aureus was the most predominant organism. Among the bacterial isolates, the presence of different types of proteolytic activities was observed as follows: proteinase K (87.2%), collagenase (80.8%), hyaluronidase (78.2%), caseinase (60.3%), alkaline protease (53.8%) and gelatinase (25.6%). Conclusions: Bacterial wound flora were found capable to produce and secrete proteolytic enzymes and it can be worsen the proper wound healing
Keywords: Caseinase, Alkaline protease, Hyaluronidase, Proteinase K, Collagenase
Full Text:
NTRODUCTION
Diabetic foot ulceration is one among the foremost complications of diabetes and 85% of amputation cases are reported because of diabetic foot ulcers. Increased incidence of infections in the wound further adds to the complications. Diabetes slows down the conventional functioning of wound healing processes resulting in vascular and neuropathic disorders . The peripheral neuropathy associated with diabetes cause the degradation of cell epithelium , serving the microbes to overcome the cell barrier of the host. Bacterial infection, along with local tissue hypoxia, ischemia, continued trauma and altered cellular and systemic stress response causes wounds to heal slowly; reworking them into chronic wounds. The colonisation of bacteria in wounds hinders the wound healing process. These pathogenic bacteria cause disease by mechanism of establishment, production of invasins and bypassing host defence mechanisms. Bacteria produce proteolytic enzymes like hyaluronidase , collagenase, gelatinase, caseinase, alkaline proteaseetcwhich damages host cells and help in spread of the pathogen. These enzymes have a very important role in worsening of wound. Bacterial protease hydrolyses the protein and peptide thus causing degradation of cell membranes and disrupting numerous biological functions. The number of diabetic people in the world is estimated to leap to 592 million in 2035 in comparison to 382 million in 2013 . Hence present study focuses on the prevalence of tissue damaging enzymes of bacterial isolates from diabetic ulcer foot and its role in delayed wound healing.
MATERIALS AND METHODS
Study population
The pus samples were collected from the wounds of 55 diabetic foot ulcer patients of Medical Trust Hospital and Diabetes Care Center, Pandalam, Kerala, with their informed consent and institutional ethical committee’s permission. All the specimens were handled and transported and aseptically to the microbiology laboratory for further testing.
Identification of organisms
The bacteria were isolated by streaking on nutrient agar plates and incubating at 37 C for 24 hr. The individual bacterial colonies were isolated and the identification was done based on standard medical microbiology laboratory procedures.
Qualitative tests for tissue degrading bacterial enzymes
The tissue degrading enzymatic potentials of bacteria like proteinase K, collagenase, hyaluronidase, caseinase, alkaline protease and gelatinase were analysed qualitatively. A collagen containing media (collagen + tyrode solution) was prepared and wells in the medium were inoculated with the test sample for collagenase test. For caseinase test casein agar plates were streaked with the each bacterial isolates and were checked for the presence of clearancezones. Bacterial cultures were inoculated into nutrient gelatin broth and incubated for 48hrs for gelatinase test. The tests for proteinase K, hyaluronidase and alkaline protease were done along with the quantitative tests.
Quantitative tests for tissue degrading bacterial enzymes
The bacterial isolates were quantitatively analysed for the enzymes such as proteinase K, collagenase, hyaluronidase, caseinase, alkaline protease and gelatinase. Isolated organisms were inoculated separately in 10 ml of broth at 37C for 24 hrs. The culture was then centrifuged and the pellet and supernatant were collected separately. The supernatant was directly assayed for secretory enzyme activity. The pellet was suspended in 1 ml phosphate buffer, sonicated and centrifuged and then used for intracellular enzyme assay. One unit of proteinase K was defined as 1 µM of tyrosine liberated at culture conditions using hemoglobin as substrate. Collagenase and caseinase assay was done according to the procedure of Mandal . One unit of enzyme activity was defined as µM of leucine liberated in 5 hrs under the culture conditions. Hyaluronidase was assayed by the method of Tolksdrof and Kass and Seastone. The alkaline protease activity was measured by the method of Meyers and Ahearn . Unit activity was defined as amount of enzyme that released 1 µmol of tyrosine/ml/minute. Gelatinase activity was done according to the method Tran and Nagano and was defined as µM of leucine liberated/ml/minute.
RESULTS
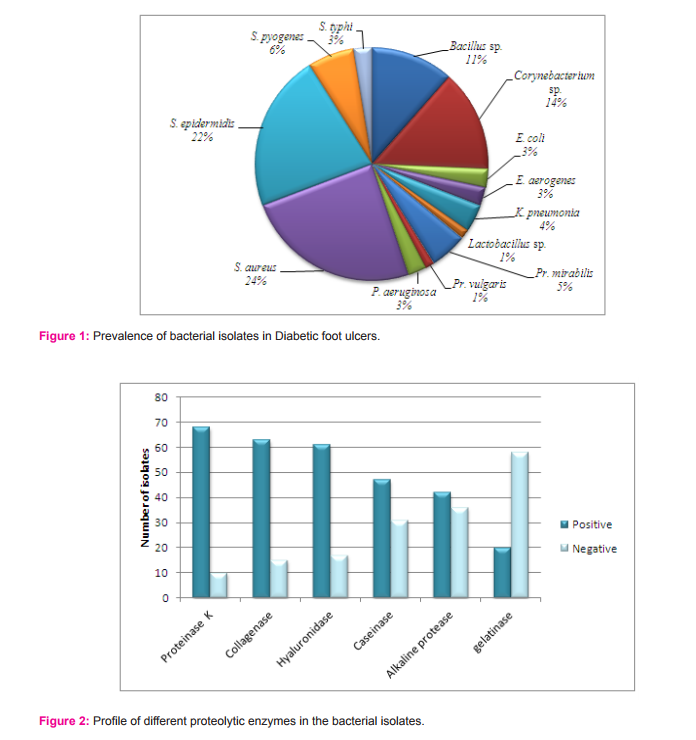
For the present study, 55 diabetic foot patients were selected those who have chronic infections of Wagner grade 2 to 5. A total of 78 strains of organisms were isolated from wound and identified biochemically. The gram positive isolates were sp., sp., and sp. The gram negative isolates were and (figure 1).
Qualitative analysis of tissue degrading proteases
The proteolyticenzymes such asproteinase K, collagenase, hyaluronidase, caseinase, alkaline protease and gelatinase were studied here to screen the proteolyticaction of bacterial flora from ulcer foot. From the qualitative analysis, it was observed that 100% of total isolates were positive for at least 2 different proteases under consideration (Figure 2), but secretory capability varies with different genus and species.
Quantitative analysis of tissue degrading proteases
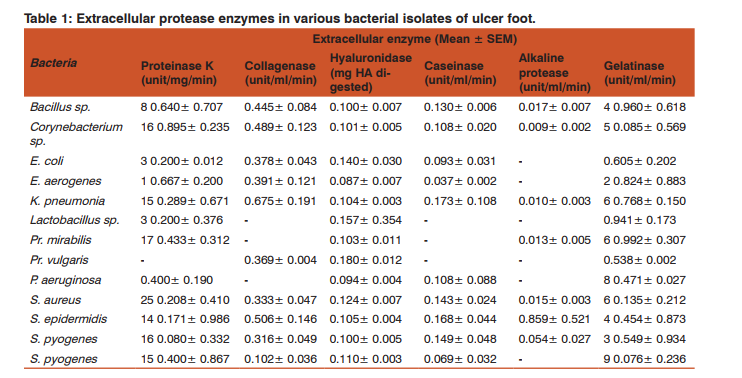
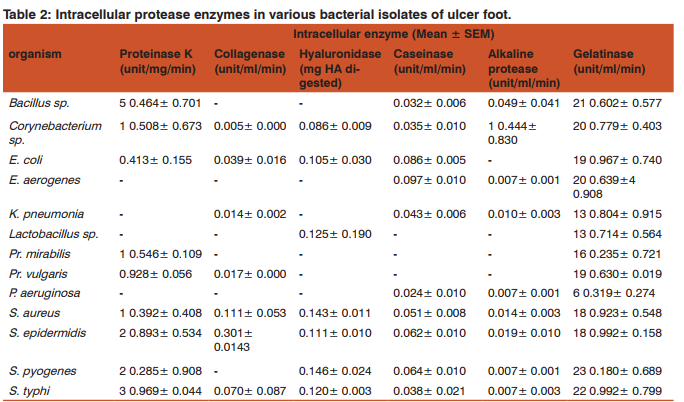
Both production and secretion of proteases were analysedby screening intracellular and extracellular enzymes respectively. Table 1 and 2 depict the level of extracellular and intracellular level of each enzyme considered in this study.
DISCUSSION
Increased infection susceptibility is seen in patients with diabetes mellitus, than non-diabetic person .The increased hyperglycemic condition leads to advanced glycation of proteins and lipids and gets deposited in the blood capillaries. This leads to the diminished blood flow and oxygen perfusion to the site of wound.The decreased oxygen condition in wound also deliver very good environment for the anaerobic organisms. Even though diabetic ulcer foot is multifactorial condition, one amongst the important reasons for non-healing wound is the tissue destruction .This tissue destruction is caused by the increased activity of the bacterial proteases. For the present study, 55 diabetic foot patients were selected those who have chronic infections. They were suffering from different grades of foot ulcer and coming under Wagner grade 2 to 5. A total of 78 strains of bacteria were isolated from wound and identified biochemically. The most predominant organisms isolated were and it accounted for 24% of the total bacterial isolates. Our previous study of bacterial isolates form diabetic foot ulcer patients admitted in hospital of Malabar region also gave a similar result of as the most predominant isolate from the wound. The predominance of in the wound isolates were reported in many other studies. This indicates the predominance of is irrespective of the locality. The degree of occurrence of other gram positive isolates other than was > sp. sp. > > sp. The order of occurrence of other gram negative isolates was > > > > > (figure 1). Hena and Growther also reported the presence of and in septic complications of infected diabetic foot patients from Coimbatore .
The wound micro-flora are capable to grow on the surface of the wound with the help of their proteolytic enzymes and other invasins like exotoxins and endotoxins . These invasins help in the spreading of the pathogen and cause considerable tissue damage. In this study, only some important invasins such as collagenase, caseinase, gelatinase, hyaluronidase, alkaline protease and proteinase K were considered. These protease enzymes can cause the destruction of extracellular matrix in chronic wounds and act as virulence factor of the infecting bacteria . Out of the total isolates, 8.97% of isolates showed activity for all the 6 enzymes taken under consideration. About 82.05% of isolates gave good activity for 50% of enzymes in our study. Among the bacterial isolates, the presence of different types of proteolytic activities was observed as follows: proteinase K (87.2%), collagenase (80.8%), hyaluronidase (78.2%), caseinase (60.3%), alkaline protease (53.8%) and gelatinase (25.6%). Both production and secretion of proteases were estimated here by screening the intracellular and extracellular enzymes respectively. The extracellular (secretory) proteases can cause extensive tissue damage , blood stream diffusion and coagulation of blood . They also help bacteria in degradation of proteins producing small peptides and amino acids which are further transported and utilized by the organism for the growth and development. Intracellular proteases help in the cellular and metabolic processes and helps in the cell to cell interaction of the organism. Depending upon the secretory potential of isolates, they showed the presence of extracellular or intracellular proteolytic activity. A group of proteolytic isolates having only extracellular activity exhibited high enzyme secretion. Another set of isolates exhibited lower extracellular activity while a third group exhibited only intracellular activity. The reason for lower or no extracellular activity can be attributed to the lack of specific substrate stimulation in condition. Regardless of the site of activity all isolates were found to be potential protease producers.
Prevalence of secretory proteases in ulcer foot
a) Bacterial proteinase K
All of proteinase K positive bacteria were found capable to produce extracellular proteinase K except Among them, showed the highest secretory potential with mean activity of 25.208±0.410 unit/ml/min. As a predominant microflora of ulcer foot, their proteinase K activity can further worsen the impaired wound healing in the infected area. Even though the infection have the prevalence next to, the proteinase K extracellular activity was lesser, but their abundance in ulcer foot can affect the protease action on tissues. While the sp. possess 8.640±0.707 unit/ml/min of extracellular activity, they have comparatively higher intracellular activity of 5.464±0.701 unit/ml/min. gave the lowest extracellular activity of 0.400±0.190 unit/ ml/min. was found negative for intracellular production of the enzyme and thereby no extracellular secretion too. According to Wandersman’s findings, the secretion of this enzyme help the each bacteria to establish infections in wound by cleaving the internal peptide bonds of proteins in normal non-diabetic condition. It also have the capability to digest the native keratin. Hence the proteinase K secretory capabilities of almost all bacterial flora can worse the tissue damage in diabetic ulcer foot.
b) Bacterial collagenase
Generally, collagenase can breakdown the peptide bonds of the collagen protein, the fibrous protein of extracellular connective tissue.Unlike human collagenase, bacterial collagenases have a broader substrate specificity . For instance, they can hydrolyze the native collagen in its triple helical conformation HShibano, YMorihara, KFukushima, JInami, SKeil, BGilles, AMKawamoto, SPOkuda, KStructural gene and complete amino acid sequence of Vibrio alginolyticus collagenaseBiochem. JBiochem. J703-7082811992, both water-insoluble native collagens and watersoluble denatured collagens and also the gelatin along with collagen as a substrate sp., and were found devoid of this enzyme. sp. and sp. showed high secretory potential of this enzyme. Maximum extracellular collagenase activity of 0.675±0.191 unit/ml/min was shown by gave the least extracellular collagenase activity of 0.102±0.036 unit/ ml/min. Most of the collagenase produced was released to extracellular environment destroying the matrix leading to delayed wound healing process and causing tissue destruction. Intracellular collagenase activity was shown by and Maximum intracellular collagenase activity of 0.301±0.0143 unit/ml/min was shown by The high rate of collagenase secretion in majority of isolates of the study reveals its capability to cause uncontrolled proteolytic tissue destruction and act as a pathogenic factor in non-healing wounds.
c) Bacterial hyaluronidase
All the isolates gave extracellular hyaluronidase activity. Most of the bacteria were capable of releasing the enzyme into the extracellular environment. The highest mean extracellular activity of 0.180±0.012 unit was shown by. They gave no intracellular activity, this might be because, the organism is capable of releasing the enzyme produced to the outside environment and thus the hyaluronidase enzyme is completely released. Least extracellular hyaluronidase activity of 0.087±0.007 unit was given by. The intracellular enzyme activity was not given by the all organism under consideration. sp., and did not show any intracellular enzyme activity. gave the highest intra cellular activity of 0.146±0.024 unit and sp. gave the lowest intracellular activity of 0.086±0.009 unit. This enzyme increases the permeability of the extracellular matrix (ECM) by hydrolysing the ECM component, the hyaluronan. Starr and Engleberg also reported hyaluronidase positive and in cellulitis in patients of United States . Some streptococcal species produces a hyaluronic acid (HA) capsule preventing phagocytosis and facilitating the adherence to the mucosal surface .This enzyme can act as virulence factor, disrupting the polysaccharides in the cell membrane and thus increasing cell wall permeability so as to promote bacterial spread.
d) Bacterial caseinase
As an important factor for virulence of bacteria isolated from wound infection, caseinase activity was also studied here. Bacterial flora of ulcer foot except and showed the presence of both extracellular and intracellular caseinase. The highest caseinase activity of 0.173±0.108 unit/ml/min was seen in the cell free supernatant. and gave the highest extracellular caseinase activity thus they can impart high proteolytic activity . exhibited the highest intracellular caseinase activity of 0.097±0.010 unit/ml/min followed by . But showed lesser ability to release enzyme in contrast to other isolates. Similarly, the presence of caseinase was detected in many hospital clinical isolates like from respiratory tract secretions, in corneal ulceration during bacterial keratitis and also in some strains. This proteolytic activity was found to be related to the pathogenesis of the bacterium and the development of nosocomial infections. Besides this enzyme is essential for the activity of haemolysin too . Therefore, the prevalence of caseinase producers in diabetic ulcer foot might have influence in delayed ulcer foot management.
e) Bacterial Alkaline protease
Alkaline protease were shown to be secreted during infection and they affect the wound healing by increasing the pH at the wound site. Normally the wound healing occurs more readily in an acidic environment of pH of 4–6. This protease enzyme elevates the pH of the wound, thus affecting many factors like oxygen release angiogenesis, protease activity bacterial toxicity etc leading the wound to remain unhealed. Alkaline protease cleaves the peptide bonds of protein and are stable at a higher pH. They are found in all living organism and are needed for the normal cell growth and differentiation . Alkaline protease activity was not observed by all the organisms taken up for the study. Organisms like sp., and gave very less alkaline protease activity. Considerable alkaline protease activity was shown only by sp. and sp. gave a maximum intracellular enzyme activity of 1.44 unit/ml/min in the sonicated cell pellet. They were unable to release the enzyme to the environment. An extracellular activity of 0.859±0.521 unit/ml/min was given by Alkaline proteases also have proteolytic activity on proteins involved in host defence mechanisms like complement activation via the classical and lectin pathways and they penetrate the body barriers and damage the host cells . They also protect the organism from the immune system of the host . Hence, bacterial alkaline protease can cause impaired wound healing in diabetic ulcer foot but, alkaline protease positive organisms was found significantly less in our study. Though the enzyme activity was less, its impact can cause severe consequences on already debilitated condition of diabetic ulcer foot.
f) Bacterial gelatinase
Generally gelatinase is capable of hydrolyzing collagen, casein, hemoglobin and other peptides.in this study, bacterial gelatinase was detected both extracellularly and intracellularly. Even though they were positive for the enzyme production, the activities of secretory gelatinase were lower than that of intracellular enzyme. was the lowest gelatinase producer in this study. Although there were contradictory reports on the positive and negative influence of human gelatinase (MMP-2 and MMP-9) in normal wound healing process, the microbial gelatinase have a negative effect on the wound healing process. They degrade the gelatin in the connective tissue and help the microorganism to further spread its infection into the tissue . Unlike other study enzymes, only 25.66% of total isolates were found as gelatinase positive. However, isolates were potential to produce gelatinase. Hence they might have role in worsening of ulcer foot in diabetic patients.
CONCLUSION
We can conclude that the bacterial infection is very common in diabetic ulcer foot. This bacterial infection has increased the burden of foot ulceration. Bacterial infection in ulcer foot may further lead to septicaemia and can result in the death of the patient. The release of proteolytic enzymes by bacteria together with the matrix metalloproteases of the host tissue causes the tissue disruption in the wound and leads to delayed wound healing. The purpose of the study was to find out the influence of different proteolytic enzymes of bacteriological origin on tissue damage and impaired wound healing process. The results of the study reveal us that the bacterial proteolytic enzymes damage the cells and tissue of the host and increase the delay of the healing process. The knowledge of the organism and its biochemical parameters helps us to provide a better treatment for the diabetic ulcer foot problem and the other poorly healing wounds.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Ethical Clearance and Informed Consent
The pus samples were collected from the wounds of diabetic foot ulcer patients of Medical Trust Hospital and Diabetes Care Center, Pandalam, Kerala, with their informed consent and institutional ethical committee’s permission.
Source of Funding
The authors acknowledge the Ministry of Human Resource Development, India, for funding of the project.
Conflict of interest
The authors have no conflict of interests.
References:
1. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease.
2. Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes care. 1999;22(7):1029-35.
3. Percival SL, Cutting KF. Microbiology of wounds. Microbiology of wounds. 2010.
4. Mustoe TA, O’Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plastic and reconstructive surgery. 2006;117(7S):35S-41S.
5. Hynes WL, Walton SL. Hyaluronidases of Gram-positive bacteria. FEMS microbiology letters. 2000;183(2):201-7.
6. Tran L, Nagano H. Isolation and characteristics of Bacillus subtilis CN2 and its collagenase production. Journal of food science. 2002;67(3):1184-7.
7. Kayaoglu G, Ørstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Critical Reviews in Oral Biology and Medicine. 2004;15(5):308-20.
8. Matsumoto K. Proteases in bacterial keratitis. Cornea. 2000;19(6):S160-S4.
9. Hoge R, Pelzer A, Rosenau F, Wilhelm S. Weapons of a pathogen: proteases and their role in virulence of Pseudomonas aeruginosa. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. 2010;2:383-95.
10. Barrett AJ, Woessner JF, Rawlings ND. Handbook of proteolytic enzymes: Elsevier; 2004.
11. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035 for the IDF Diabetes Atlas. Diabetes Research and Clinical Practice.
12. Cowan ST, Steel KJ, Barrow G, Feltham R. Cowan and Steel’s manual for the identification of medical bacteria: Cambridge university press; 2004.
13. Galloway DR. Role of exotoxins in the pathogenesis of P. aeruginosa infections. Pseudomonas aeruginosa as an Opportunistic Pathogen: Springer; 1993. p. 107-27.
14. Vasil M, Pritchard AE, Ostroff R. Molecular biology of exotoxin A and phospholipase C of Pseudomonas aeruginosa. 1990.
15. Mandl I, MacLennan JD, Howes EL, DeBellis RH, Sohler A. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. Journal of Clinical Investigation. 1953;32(12):1323.
16. Tolksdorf S, McCready M. The turbidimetric assay of hyaluronidase. The Journal of laboratory and clinical medicine. 1949;34(1):74.
17. Kass EH, Seastone C. The role of the mucoid polysaccharide (hyaluronic acid) in the virulence of group A hemolytic streptococci. The Journal of experimental medicine. 1944;79(3):319-30.
18. Meyers S, Ahearn D. Extracellular proteolysis by Candida lipolytica. Mycologia. 1977:646-51.
19. Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes care. 2003;26(2):510-3.
20. Wheat LJ. Infection and diabetes mellitus. Diabetes care. 1980;3(1):187-97.
21. Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. Jama. 2002;288(20):2579-88.
22. Wunderlich RP, Peters EJ, Lavery LA. Systemic hyperbaric oxygen therapy: lower-extremity wound healing and the diabetic foot. Diabetes Care. 2000;23(10):1551-5.
23. Falanga V. Wound healing and its impairment in the diabetic foot. The Lancet. 2005;366(9498):1736-43.
24. Miyoshi S-i, Shinoda S. Microbial metalloproteases and pathogenesis. Microbes and infection. 2000;2(1):91-8.
25. Mathew SM, Suchithra TV. A Threatening Approach of Wound Microflora to Diabetic Ulcer Foot Management. Int J Curr Microbiol App Sci. 2014;3(9):640-6.
26. Tentolouris N, Petrikkos G, Vallianou N, Zachos C, Daikos GL, Tsapogas P, et al. Prevalence of methicillin-resistant Staphylococcus aureus in infected and uninfected diabetic foot ulcers. Clin Microbiol Infect. 2006;12(2):186-9.
27. Orji F, Nwachukwu N, Udora E. Bacteriological evaluation of diabetic ulcers in Nigeria. African Journal of Diabetes Medicine. 2009;15(11):19-21.
28. Hena J, Growther L. Studies on bacterial infections of diabetic foot ulcer. African Journal of Clinical and Experimental Microbiology. 2010;11(3).
29. Srivastava S, Srivastava P. Bacteria and Huma30. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9(1):283-9.
31. Rahme LG, Ausubel FM, Cao H, Drenkard E, Goumnerov BC, Lau GW, et al. Plants and animals share functionally common bacterial virulence factors. Proceedings of the National Academy of Sciences. 2000;97(16):8815-21.
32. Kastrup CJ, Boedicker JQ, Pomerantsev AP, Moayeri M, Bian Y, Pompano RR, et al. Spatial localization of bacteria controls coagulation of human blood by’quorum acting’. Nature chemical biology. 2008;4(12):742-50.
33. Staats CC, Boldo J, Broetto L, Vainstein M, Schrank A. Comparative genome analysis of proteases, oligopeptide uptake and secretion systems in Mycoplasma spp. Genetics and Molecular Biology. 2007;30(1):225-9
. 34. An S-Y, Ok M, Kim J-Y, Jang M-S, Cho Y-S, Choi Y-L, et al. Cloning, high-level expression and enzymatic properties of an intracellular serine protease from Bacillus sp. WRD-2. Indian Journal of Biochemistry And Biophysics. 2004;41:141-7.
35. Wandersman C. Secretion, processing and activation of bacterial extracellular proteases. Molecular microbiology. 1989;3(12):1825-31.
36. Nam G-W, Lee D-W, Lee H-S, Lee N-J, Kim B-C, Choe E-A, et al. Native-feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Archives of Microbiology. 2002;178(6):538-47.
37. Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, et al. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. The EMBO journal. 2004;23(15):3020-30.
38. Toyoshima T, Matsushita O, Minami J, Nishi N, Okabe A, Itano T. Collagen-binding domain of a Clostridium histolyticum collagenase exhibits a broad substrate spectrum both in vitro and in vivo. Connective tissue research. 2001;42(4):281-90.
39. Takeuchi H, Shibano Y, Morihara K, Fukushima J, Inami S, Keil B, et al. Structural gene and complete amino acid sequence of Vibrio alginolyticus collagenase. Biochem J. 1992;281:703-8.
40. Mookhtiar K, Van Wart H. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix (Stuttgart, Germany) Supplement. 1991;1:116-26.
41. Metzmacher I, Ruth P, Abel M, Friess W. In vitro binding of matrix metalloproteinase-2 (MMP-2), MMP-9, and bacterial collagenase on collagenous wound dressings. Wound repair and regeneration. 2007;15(4):549-55.
42. Starr CR, Engleberg NC. Role of hyaluronidase in subcutaneous spread and growth of group A streptococcus. Infection and immunity. 2006;74(1):40-8.
43. Wessels MR, Bronze MS. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proceedings of the National Academy of Sciences. 1994;91(25):12238-42.
44. Smith NL, Taylor EJ, Lindsay A-M, Charnock SJ, Turkenburg JP, Dodson EJ, et al. Structure of a group A streptococcal phage-encoded virulence factor reveals a catalytically active triple-stranded β-helix. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17652-7.
45. Žukaite V, Biziulevi?ius G. Acceleration of hyaluronidase production in the course of batch cultivation of Clostridium perfringens can be achieved with bacteriolytic enzymes. Letters in applied microbiology. 2000;30(3):203-6.
46. Garcia DdO, Timenetsky J, Martinez MB, Francisco W, Sinto SI, Yanaguita RM. Proteases (caseinase and elastase), hemolysins, adhesion and susceptibility to antimicrobials of Stenotrophomonas maltophilia isolates obtained from clinical specimens. Brazilian Journal of Microbiology. 2002;33(2):157-62.
47. Furumura MT, Figueiredo P, Carbonell GV, Darini ALdC, Yano T. Virulence-associated characteristics of Enterococcus faecalis strains isolated from clinical sources. Brazilian Journal of Microbiology. 2006;37(3):230-6.
48. Sheeran B, Smith P. A second extracellular proteolytic activity associated with the fish pathogen Aeromonas salmonicida. FEMS Microbiology Letters. 1981;11(1):73-6.
49. Percival SL, Cochrane CA. Wounds, Enzymes, and Proteases. Microbiology of Wounds. 2010:249.
50. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Archives of dermatological research. 2007;298(9):413-20.
51. Gethin G. The significance of surface pH in chronic wounds. Wounds uk. 2007;3(3):52.
52. Syed R, Roja Rani S, Masoodi TA, Shafi G, Alharbi K. Functional analysis and structure determination of alkaline protease from Aspergillus flavus. Bioinformation. 2012;8(4):175.
53. Laarman AJ, Bardoel BW, Ruyken M, Fernie J, Milder FJ, van Strijp JA, et al. Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. The Journal of Immunology. 2012;188(1):386- 93.
54. Kernacki K, Hobden J, Hazlett L, Fridman R, Berk R. In vivo bacterial protease production during Pseudomonas aeruginosa corneal infection. Investigative ophthalmology and visual science. 1995;36(7):1371-8.
55. Saleem AJ. Relationship Study between the Alkaline Protease Production and the Growth Phases of Pseudomonas aeruginosa Isolated from Patients. Advances in Microbiology. 2012;2(03):354.
56. Upadhyaya PG, Umapathy B, Ravikumar K. Comparative study for the presence of enterococcal virulence factors gelatinase, hemolysin and biofilm among clinical and commensal isolates of Enterococcus faecalis. Journal of laboratory physicians. 2010;2(2):100.
57. Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. Journal of Biological Chemistry. 2002;277(3):2065-72.
58. Ladwig GP, Robson MC, Liu RAN, Kuhn M, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase†9 to tissue inhibitor of matrix metalloproteinase†1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair and Regeneration. 2002;10(1):26-37.
59. Lopes MdFS, Simões AP, Tenreiro R, Marques JJF, Crespo MTB. Activity and expression of a virulence factor, gelatinase, in dairy enterococci. International journal of food microbiology. 2006;112(3):208-14.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License