IJCRR - 7(12), June, 2015
Pages: 24-30
Print Article
Download XML Download PDF
HAEMORHEOLOGY AND RED CELL INDICES IN HIV POSITIVE INDIVIDUALS ON ANTIRETROVIRAL
THERAPY IN DELTA STATE, NIGERIA
Author: Ifeanyichukwu Martin Ositadinma*, Osakue Stanley Ikponmwosa, Okeke Chizoba Okechukwu
Category: Healthcare
Abstract:Objective: This work was designed to study the impact of HIV and anti-retroviral therapy on some haematological and haemorheological parameters. Method: Two hundred and fifty three subjects aged 18 \? 60 years; comprising; 85 HIV patients on ART, 90 not on ART and 78 age-matched apparently healthy HIV negative controls were recruited. CD4+ count, Haematocrit, Mean corpuscular volume (MCV), Mean corpuscular haemoglobin (MCH), Mean corpuscular haemoglobin concentration (MCHC), red cell distribution width (RDW), Whole blood viscosity (WBV), plasma viscosity (PV) and plasma fibrinogen were assayed. Result: WBV, PV, plasma fibrinogen, RDW and MCV were significantly higher in HIV-positive subjects on ART and those not on ART compared to the controls (p< 0.05). While HCT and CD4+ count, were significantly lower in HIV-positive subjects on ART and those not on ART compared to the controls (p< 0.05). MCH and CD4 count were significantly higher in HIV subjects on ART than non-ART subjects (P< 0.05). MCV was significantly higher in female than males on ART and non-ART (P< 0.05). HCT was significantly higher in males than females in subjects not on ART and the control subjects (P< 0.05). Plasma fibrinogen was significantly higher in females than males on ART and control subjects while MCH and CD4 count were higher in male than female control subjects (P< 0.05 respectively). Conclusion: The increase in WBV, PV and Plasma fibrinogen indicates an altered haemorheology while the decrease in HCT, MCH and MCHC is a pointer to anaemia known to be prevalent in HIV patients. The reduction in CD4 indicates the immunosuppressed
state of the patients.
Keywords: Haemorrheology, Red cell indices, Antiretroviral therapy, HIV
Full Text:
INTRODUCTION
Since its identification in 1981, Human immunodeficiency virus (HIV) infection and associated Acquired immune deficiency syndrome (AIDS) remain a major burden globally [1]. Nigeria has the second highest number of people living with Human immunodeficiency virus (3.1 million) next to South Africa (5.6 million). It accounts for 10% of the global Human immunodeficiency virus burden. In patients infected with Human immunodefi- In patients infected with Human immunodeficiency virus, the prevalence of cardiovascular risk factors is greater than in the general population. Both the Human immunodeficiency virus infection itself and the anti-retroviral treatment play a role in the development of cardiovascular event [2]. Haemorheology is the study of the flow properties of blood and its elements. There is increasing evidence that flow properties of blood are among the main determinants of proper tissue perfusion, and alteration in these properties play significant role in disease processes. Whole blood viscosity is the property of the fluidity and internal friction of blood. Increased viscosity may be one mechanism by which all major risk markers may promote cardiovascular disease [3]. Generally, changes in whole blood viscosity have been reported in several human cardiovascular diseases indicating that blood viscosity may be a major cardiovascular risk factor. Men have been shown to have higher blood viscosity than women, largely because of their higher hematocrit. Plasma viscosity is the intrinsic flow resistance of plasma. Increased plasma viscosity and whole-blood viscosity are observed in primary hyperlipoproteinemias as well as in secondary hyperlipoproteinemias such as diabetes mellitus and the nephrotic syndrome [3]. Plasma viscosity is primarily dependent on the concentration of plasma proteins, especially fibrinogen and it is not affected by anaemia [4]. According to [5], elevated plasma fibrinogen levels may be regarded as an independent cardiovascular risk factor. Cardiovascular disease is associated with high fibrinogen and lipid fractions leading to an increase of both plasma and whole blood viscosity as well as raised aggregability of blood cells [6]. The Haematologic manifestations of Human immunodeficiency virus infection are well-recognized as major complication of the disease and may be clinically important in many patients. The physiopathology of Human immunodeficiency virus-associated anaemia may involve three basic mechanisms: decreased Red Blood Cell production, increased Red Blood Cell destruction, and ineffective Red Blood Cell production. Red cell distribution width is an automated measure of the heterogeneity of red blood cell sizes (e.g. anisocytosis) and routinely performed as part of a complete blood cell counts. Red cell distribution width is used in the differential diagnosis of anemia. Red cell distribution width which also indicates the degree of anisocytosis is currently considered a new marker of inflammatory activity. A high level of red blood cell distribution width is a novel prognostic marker that may reflect an underlying inflammatory state. Recently, a series of studies have demonstrated that red blood cell distribution width can serve as a novel, independent predictor of prognosis in patients with cardiovascular diseases [7]. This study was therefore aimed at studying the impact of Human immunodeficiency virus infection and anti-retroviral therapy on some haematological and haemorheological parameters of Human immunodeficiency virus patients.
MATERIALS AND METHOD
SUBJECTS SELECTION
A total of one hundred and seventy-five (175) HIV-positive subjects (60 males and115 females) between the ages of 18-60yrs were recruited for this study from the Voluntary Counselling and Testing (VCT) centre in Delta State Government Hospital and Sage Clinic both in Warri, Delta State. Eighty five (85) were on Anti-retroviral drugs and Ninety (90) were not on anti-retroviral therapy. Seventy-eight (78) apparently healthy age-matched HIV sero-negative participants were also recruited as controls (30 males and 48 females). Ethical approval was obtained from the Ethics committee of Delta State Hospital Management board and informed consent was obtained from all participants that were involved in this study. Inclusion criteria were HIV positive patients on antiretroviral therapy (ART) at the duration of three months to thirty-eight months, HIV positive patient not on ART and HIV negative patients as control subjects. Exclusion criteria were Pregnant women, subjects with known bleeding or clotting disorders, including history of deep vein thrombosis, Concurrent malignancy requiring cytotoxic chemotherapy or radiation therapy, Patient less than 18years or above 60 years of age and subjects that declined from participating in the study.
SAMPLE COLLECTION
Six (6) millilitres of blood was collected from the antecubital fossa of each subject, 4ml was dispensed into EDTA container for CD4 count, haematological and haemorheological parameters, the remaining 2ml into plain containers (chemically clean plastic tube), which was allowed to clot and serum used for serum viscosity and HIV screening.
METHODS
HIV 1/2 RAPID TEST KIT BY IMMUNOCHROMATOGRAPHY
Determine HIV ½ test strips (Alere medical company Ltd, Japan, 2013, lot No: 53427K100) Fifty microliter (50µL) of plasma sample was dispensed into the specimen pad of the test strip. The reaction was allowed for 15 minutes. The appearance of distinct red lines on the test and control regions of the kit suggests a positive HIV test. While only one distinct red line in the control region suggested a negative result. Appearance of the distinct red line on the control region will validates the result without which the kit was assumed non-functional.
STAT PAK HIV ½ test kit (CHEMBIO DIAGNOSTIC SYSTEMS INC., USA 2012, lot No: HIV070612)
The test kit was placed on a flat surface; 5µL sample loop provided for the specimen was used to touch the sample allowing the opening of the loop to fill with plasma which was then placed into the sample pad. Three (3) drops of the buffer drop-wise was added to the sample in the sample well. Result was read after 10 minutes of adding the buffer.
UNI-GOLD HIV ½ test kit (TRINITY BIOTECH PLC, Ireland 2013, lot HIV3100204)
The test device was removed from the protective wrapper. Over the sample port 60µL of serum was added. Also two drops of wash buffer reagent was added to the sample on its port. Result was read after 10 minutes. CD 4+ T CELL COUNT (Partec, Germany) Twenty microlitre (20µL) sequestrated blood was collected into a partec test tube (Rohren tube). Then 20µL of CD4+ T cell antibody will be added into same tube. The content was mixed and incubated in the dark for 15 minutes at room temperature. 800µL of CD4 buffer was then added to the mixture in the tube and mixed gently. The partec tube was then plunged on the Cyflow counter and the CD4+ T cell count displayed as peak and interpreted as figures.
RED CELL INDICES (using DIRUI BCC-3000B
Auto Haematology Analyser) Haematocrit (HCT), Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH), Mean Corpuscular Haemoglobin Concentration (MCHC) and Red Cell Distribution Width (RDW) was analysed using DIRUI BCC-3000B Auto Haematology Analyser. The procedure was according to manufacturers’ instruction.
WHOLE BLOOD VISCOCITY AND PLASMA VISCOSITY
Whole Blood Viscosity (WBV) and Plasma Viscosity (PV) were carried out by a modification of the method of Reid and Ugwu [8].
PLASMA FIBRINOGEN ESTIMATION
Fibrinogen was estimated using the method of miller et al [9].
STATISTICAL ANALYSIS
The results were statistically analysed using the Statistical Package for Social Sciences (SPSS) version 21. Data were expressed as mean ± SD. Analysis of variance (ANOVA) was used to compare differences among groups, while student t-test was used to compare the differences between groups. Values were considered significant at P<0.05.
RESULTS
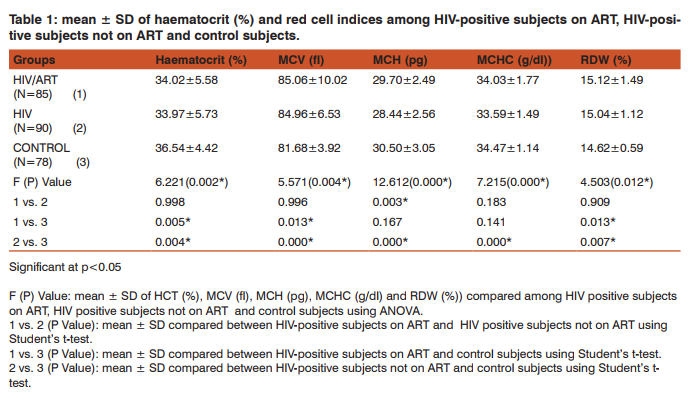
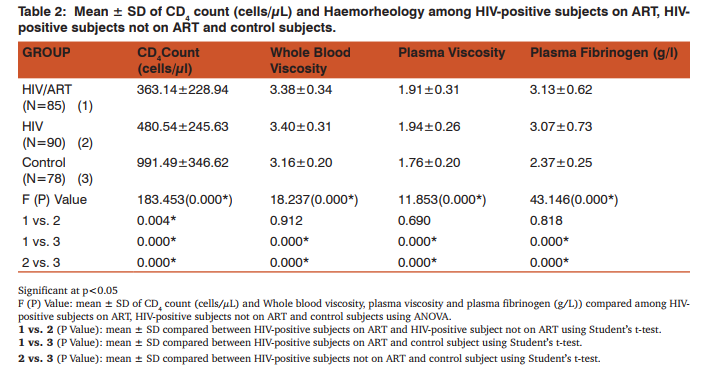
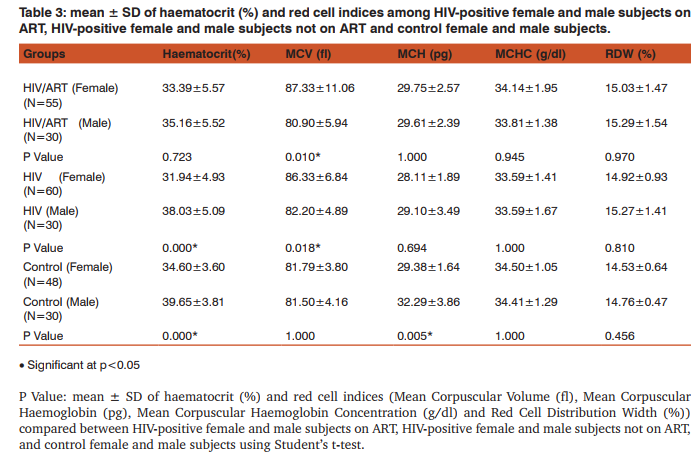
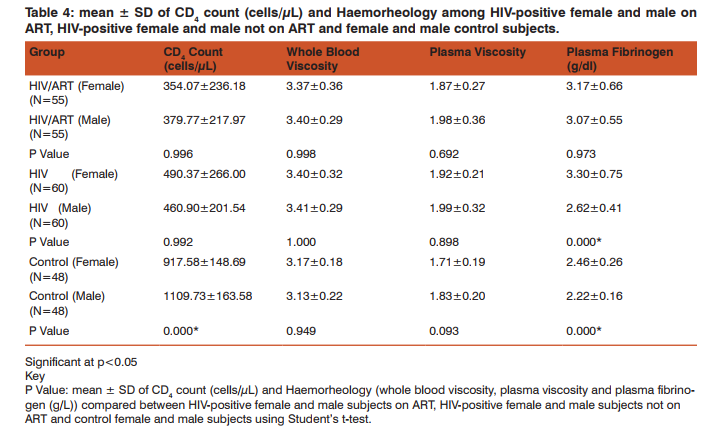
The mean values of HCT (%) was significantly lower in HIV-positive subjects (ART and non-ART) compared with control subjects (p<0.05). Similarly, mean ± S.D RDW (%) was significantly higher in HIV-positive subjects (ART and non-ART) compared with control subjects (p<0.05). The mean of MCV (fl) in HIV-positive subjects (ART and non-ART) was significantly higher than control subjects (p<0.05). Similarly, MCH (pg) and MCHC (g/ dl) of HIV-positive subjects on ART and non-ART were significantly lower compared with corresponding values in the control (p<0.05). The mean ± S.D of MCH (pg) was significantly higher in HIV-positive on ART compared with the corresponding values in HIV-positive not on ART (p<0.05) (Table 1). CD+ count (cells/µL) was significantly lower in HIV-positive subjects (ART and non-ART) compared with control subjects (p<0.05). Similarly, the mean values of whole blood viscosity, plasma viscosity and plasma fibrinogen were significantly higher in HIV-positive subjects (ART and non-ART) compared with control subjects (p<0.05). The mean of CD+ count (cells/µL) was significantly higher in HIV-positive subjects on ART compared with HIVpositive subjects not on ART (p<0.05) (Table 2). The mean ± S.D of MCV was significantly higher in female subjects on ART than the male subjects. Similarly, in non-ART subjects, HCT was significantly higher in males than females while MCV was higher in males than females non-ART subjects. Moreover, HCT and MCV were significantly higher in males than the female controls (Table 3). There was a significantly higher mean values in plasma fibrinogen (g/L) when compared between HIV-positive female and male subjects not on ART as well as in the control subjects. (p<0.05). The mean values of CD4 count was significantly higher in male controls compared to the female controls (P<0.05) (Table 4).
DISCUSSION
Haematological abnormalities are among the most common abnormalities of HIV infection which involves all lineages of blood cells [10]. HIV infection affects haemato HIV infection affects haematological indices of patients regardless of age, sex and ART [11]. This study generally observed a significant increase in the MCV and RDW as well as a significant decrease in HCT, in HIV-positive subject on ART and HIV-positive subjects not on ART compared to the control subjects, while MCH and MCHC was significantly decreased in non-ART subjects compared to the control. These might be suggestive of macrocytic hypochromic anaemia. The cause of HIV-related anaemia is known to be multi-factorial. It may result from the indirect effects of HIV infection, adverse reactions to medications, opportunistic infections or neoplasm; nutritional causes such as anorexia or malabsorption; or metabolic disorders associated with HIV [12]. HIV may directly affect bone marrow stromal cells, leading to decreased production of red blood cells and other bone marrow elements [13]. Other study showed that soluble factors like HIV proteins and cytokines may inhibit the growth of haematopoietic cells in the bone marrow [14]. Moreover, MCH was significantly higher in HIV-positive subjects on ART than those not on ART this serves to confirm the effectiveness of ART in improving the quality of life of HIV patients. HIV-positive patients (on ART and non-ART) had a reduced haematocrit compared to the control. This confirms the preponderance of anaemia in HIV patients. Haematocrit was significantly lower in HIV-positive female subjects not on ART than their male counterpart. This is in agreement with previous findings [15]. HCT was also lower in female than male control; this could be due to the fact that males normally have a higher HCT than females. MCV was significantly higher in HIV patients (ART and non-ART) than control, signifying the likelihood of developing macrocytic anaemia. This is supported by a previous finding that reported low levels of vitamin B12 [16] and folate deficiency [17, 18] in HIV infected patients. Elevated MCV could be associated with the use of zidovudine (AZT) or stavudine (d4T) [19]. Thus the elevated MCV observed in patients on ART in this study could therefore be attributed to antiretroviral drug use. MCV was also significantly higher in females than males in both ART and those not on ART. The reason for this is not apparent. RDW was observed to be significantly higher in HIV-positive subject on ART and those not on ART compared with the control subjects. This could be as a result of nutritional deficiency of various causes in this group. Previous studies [20, 21], also attributed these high values of RDW to an underlying inflammatory state that leads to impaired erythrocyte maturation and anisocytosis. This can exacerbate oxidative and inflammatory stress, which is a potential mechanism for the increased cardiovascular risk in this condition [22, 23]. CD4 T- cell count serves as the major clinical indicator of immune-competence in patients with HIV infection and hence usually the most important consideration in decision to initiate ART [24]. This study showed a significant reduction in CD4 count in HIV-positive subjects both on ART and those not on ART compared with the control subjects. This could be explained by the finding that HIV attacks and destroys cells with the CD4 antigen [25]. Altered blood flow is believed to contribute to microvascular disease and thus, studying the rheological behaviour of formed elements in the blood may provide insight into the pathogenesis of the microvascular abnormalities associated with HIV disease. Whole blood viscosity, plasma viscosity, haematocrit and plasma fibrinogen are known to influence in vivo blood flow. In this study, there were significant increase in whole blood viscosity, plasma viscosity, plasma fibrinogen in HIV-positive subjects on ART and those not on ART compared to the control subjects. Similarly, there was a significantly higher value of plasma fibrinogen in female control and in subjects not on ART compared with the males. ART has been known to improve immunity and haematocrit values [26]. Thus, the placement of these patients on ART may improve their haematocrit value and in turn improve their whole blood viscosity. The increase plasma viscosity observed among HIV-positive patients in this study correlates with the observed increase in the plasma fibrinogen concentration. Fibrinogen concentration significantly affects plasma viscosity. These findings agree with earlier reports [25, 27, 28]. Clinically an increase in plasma fibrinogen (and by extension plasma viscosity) is a risk factor for Cardiovascular disease [29, 30].
CONCLUSION
Conclusively, HIV and ART have effects on both the haematological and haemorheological parameters of HIV-positive patients. The increase in WBV, PV and Plasma fibrinogen indicates an altered haemorheology which is a risk factor for cardiovascular abnormalities in HIV patients while the decrease in HCT, MCH and MCHC is a pointer to anaemia known to be prevalent in HIV patients. The reduction in CD4 indicates immunosuppressant in the patients.
RECOMMENDATION
HIV-positive subject should therefore be assessed for haemorheologic (whole blood and plasma viscosity and plasma fibrinogen), haematologic (full blood count with red cell indices inclusive) before enrolment for HAART and periodically during therapy given their incidence, potential for morbidity and possible cardiovascular risk. Viral load assay with haemorheology is necessary to ascertain possible effect of viraemia on these parameters.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
SOURCE OF FUNDING
This research work was entirely self-funded and did not receive funding from government or public and private funding agencies.
CONFLICT OF INTEREST
The authors declare that no conflict of interest exists in this research work.
References:
1. Dapper V, Emem-Chioma P, Didia B. Some haematological parameters and the prognostic value of CD4, CD8 and total lymphocyte counts and CD4/CD8 cells count ratio in healthy HIV sero-negative, healthy HIV sero-positive and AIDS subjects in Port Harcourt, Nigeria. Turkish Journal of Haematology 2008; 25:182-186.
2. Gallego ML, Perez-Hernandez IA, Palacios R, Ruiz-Morales J, Nuno E, Marquez M, Santos J. Red cell distribution width in patients with HIV infection. Open Journal of Internal Medicine 2012; 2: 7-10.
3. Lowe GD. Blood Viscosity, Lipoproteins, and Cardiovascular Risk. Circulation 1992; 85 : 2329-2331
4. Lewis MS, Bain BJ, Bates J. Miscellaneous tests, Practical Haematology, (10th Edition). Churchill Livingstone, Elsevier. 2011; P 600.
5. Koenig W, Sund M, Ernst E, Mraz W, Hombach V, Keil U. Association between rheology and components of lipoproteins in human blood. Results from the MONICA project. Circulation 1992; 85: 2197-2204.
6. Walzl M, Schied G, Walzl B. Effect of ameliorated haemorheology on clinical symptoms in cerebrovascular disease. Arteriosclerosis 1998; 139:385-389.
7. Puerta S, Gallego M, Palacios R, Ruiz J, Nuño E, Márquez M, Santos J. Higher red blood cell distribution width is associated with a worse virologic and clinical situation in HIV-infected patients. Biomed Central 2010; 13: 69
8. Reid H, Ugwu AC. Simple technique of rapid determination of plasma and whole blood viscosity. Nigeria Journal of Physiological Sciences 1987; 3:45-48.
9. Millar HR, Simpson JG, Stalker AL. An evaluation of the heat precipitation method of plasma fibrinogen estimation. Journal of clinical Pathology 1971; 24: 827-830.
10. Tagoe DN, Asantewaa E. Profiling Haematological Changes in HIV Patients Attending Fevers Clinic at the Central Regional Hospital in Cape Coast, Ghana: A Case-Control Study. Archives of Applied Science Research 2011; 3: 326- 331.
11. Obirikorang C, Yeboah FA. Blood haemoglobin measurement as a predictive indicator for the progression of HIV/ AIDS in resource-limited setting. Journal of Biomedical Science 2009; 16: 102-109.
12. Akinbami A, Oshinaike O, Adeyemo T, Adediran A, Dosunmu O, Dada M, Durojaiye I, Adebola A, Vincent O. Haematologic Abnormalities in Treatment-Naïve HIV Patients. Infectious Diseases: Research and Treatment 2010; 3: 45–49.
13. Tripathi AK, Misra R, Kalra P, Gupta N, Ahmad R. Bone Marrow Abnormalities in HIV Disease. Journal of the Association of Physicians of India 2005; 53: 705-711.
14. Alem M, Kena T, Baye N, Ahmed R, Tilahun S. Prevalence of Anaemia and Associated Risk Factors among Adult HIV Patients at the Anti-Retroviral Therapy Clinic at the University of Gondar Hospital, Gondar, Northwest Ethiopia. Open Access Scientific Reports 2013; 2: 6-11.
15. Ferede G, Wondimeneh Y. Prevalence and related factors of anaemia in HAART-naive HIV positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Haematology 2013; 13:8-16
16. Burkes RL, Cohen H, Krailo M, Sinow RM, Carmel R. Low serum Cobalamin levels occur frequently in the acquired immune deficiency syndrome and related disorders. European Journal of Haematology 1987; 38:141-147.
17. Beach RS, Mantero-Atienza E, Eisdorfer C, Fordyce-Baum MK. Altered folate metabolism in early HIV infection. Journal of American Medical Association 1988; 259:519.
18. Boudes P, Zittoun J, Sobel A. Folate, vitamin B12, and HIV infection. Lancet 1990; 335:1401-1402.
19. Moyle G. (2002). Anaemia in persons with HIV infection: prognostic marker and contributor to morbidity. AIDS Rev. 4:13-18.
20. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJV, Pfeffer MA, Swedberg K, Yusuf S, Michelson EL, Granger CB. Red cell distribution width as a novel prognostic marker in heart failure: data from CHARM program and Duke data bank. Journal of American College of Cardiology 2007; 50: 40–47
21. Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. For the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008; 117:163–168.
22. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Archive of Pathology and Laboratory Medicine 2009; 133: 628–632.
23. Sánchez-Chaparro MA, Calvo-Bonacho E, González-Quintela A, Cabrera M, Sáinz JC, Fernández-Labandera C, Aguado LQ, Meseguer AF, Valdivielso P, Román-García J. Higher Red Blood Cell Distribution Width Is Associated With the Metabolic Syndrome, Diabetes Care 2010; 33: 40
24. Ntekim AI, Folasire AM. CD4 Count and Anti-Retroviral Therapy for HIV Positive Patients with Cancer in Nigeria -A Pilot Study. Clinical Medicine Insights: Oncology 2010; 4: 61–66
25. Omoregie R, Omokaro EU, Palmer O, Ogefere HO, Egbeobauwaye A, Adeghe J, Osakue SI, Ihemeje VI. Prevalence of anaemia among HIV infected patients in Benin City, Nigeria. Tanzania Journals of Health Research 2009; 11: 1-5.
26. Odunukwe N, Idigbe O, Kanki P, Adewole T, Onwujekwe D, Audu R, Onyewuche J. Haematological and biochemical re-sponse to treatment of HIV-1 infection with a combination of nevirapine + stavudine + lamivudine in Lagos, Nigeria. Turkish Journal of Haematology 2005; 22:125–131.
27. Winkins EG, Fraser I, Barnes A, Khoo S, Hamour A. Plasma Viscosity in HIV-infection. International Conference on AIDS 1992; 8: 143.
28. Kim A, Dadgostar H, Holland GN, Wenby R, Yu F, Terry GB, Meiselman HJ. Haemorheological Abnormalities Associated with HIV infection: Altered Erythrocyte Aggregation and deformability. Investigative Ophthalmology and Visual Science 2006; 47: 3927-3932.
29. Walzl M, Schied G, Walzl B. Effect of ameliorated haemorheology on clinical symptoms in cerebrovascular disease. Arteriosclerosis 1998; 139:385- 389.
30. Rashid SK, Bashir L. Plasminogen and fibrinogen plasma level in coronary artery disease. Revista Brasileira De Haematologia E Hemoteropia 2012; 34: 254-262.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License