IJCRR - 7(15), August, 2015
Pages: 26-31
Date of Publication: 11-Aug-2015
Print Article
Download XML Download PDF
INVASIVE LOBULAR BREAST CARCINOMA (ILC) IN AN ELDERLY MALE ON PROLONGED RANITIDINE THERAPY
Author: Srikantaiah Hiremath, Nikhil Nanjappa B. A.
Category: Healthcare
Abstract:Breast carcinoma in men is an exceedingly rare entity, contributing to 1% of all breast cancers [1], of which invasive lobular carcinoma (ILC) accounts for 1.5% [2]. The average age at diagnosis is usually five years older compared to women [1, 3]. Although the exact aetiology remains unknown, hyperestrogenic states are attributed as the most likely cause [4]. Men with family history of female breast cancer are at 2.5 times more increased risk when compared to those with no family history [5]. Lobular histologic subtype of breast carcinoma in males is rare due to the lack of acini and lobules [6]. Some medications, such as cimetidine
[7, 8] are thought to multiply the risk of male breast cancer because they induce a hyperestrogenic state. The authors report a rare case of ILC in a 75 year old man, with no known risk factors for breast cancer. However, he had a history of prolonged self-prescribed ranitidine use for dyspeptic symptoms. Although there isn't enough evidence to suggest that ranitidine, like its predecessor cimetidine, may cause male breast cancer. The authors would like to draw attention to this possibility.
Keywords: Male breast carcinoma, Male breast cancer, Invasive lobular carcinoma males, Ranitidine induced male breast cancer
Full Text:
INTRODUCTION
Breast carcinoma in men is an exceedingly rare entity. As the incidence is very low, the exact aetiology remains unknown; however, it is largely attributed to oestrogen excess states. Men who have family history of female breast cancer are at 2.5 times increased risk when compared to those with no family history [5]. The average age at diagnosis is five years older compared to women [1, 9]. Most of the breast cancers in men are of ductal (in situ and invasive) type. Invasive lobular cancer is an extremely rare histopathological type of male breast cancer because lobular and acinar components are rarely found in the normal male breast [10]. Breast cancer in men carries a worse prognosis when compared to women, although the principles of management remain the same. We present an extremely rare case of invasive lobular carcinoma (ILC) in an elderly gentleman with no other risk factors but for prolonged ranitidine therapy. Although there is not enough evidence to suggest that ranitidine, like its predecessor cimetidine, may cause male breast cancer, the authors would like to draw attention to this possibility.
CASE REPORT
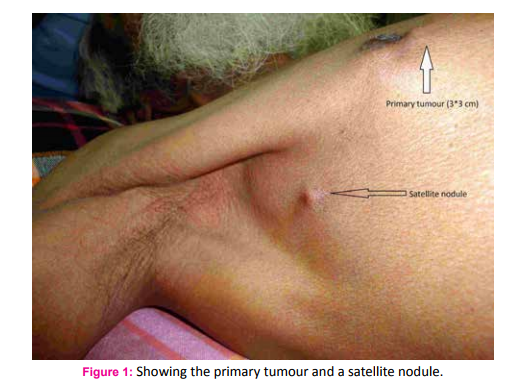
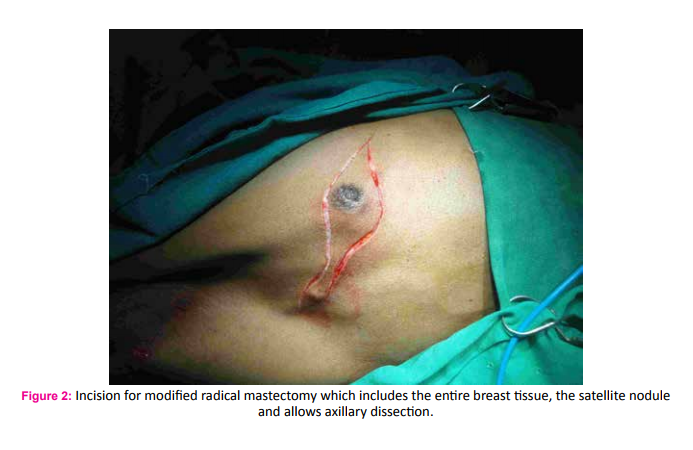
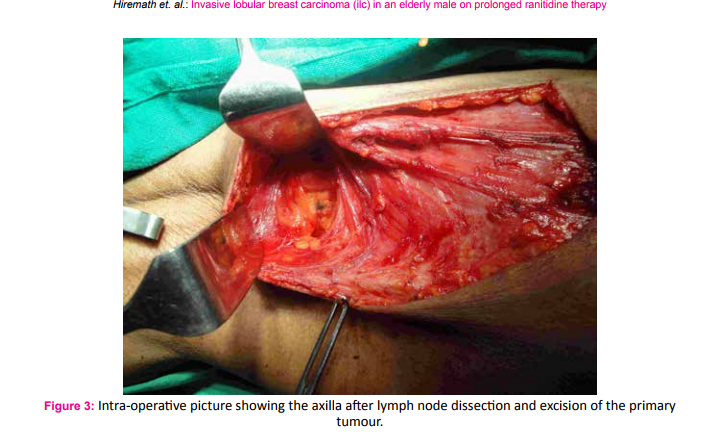
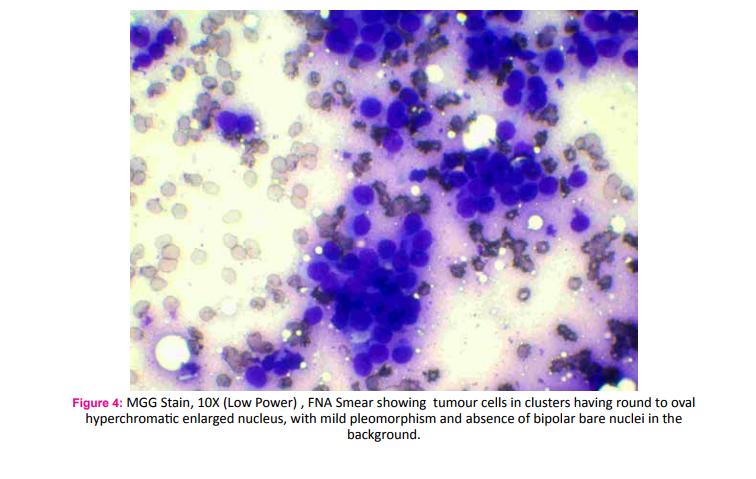
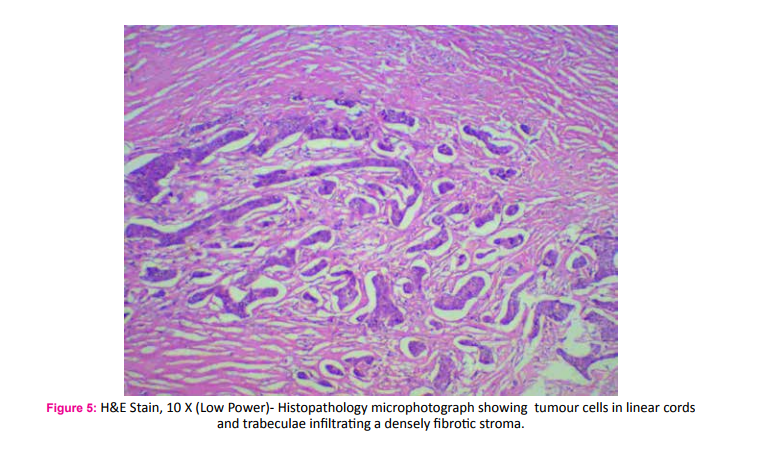
A 75 year old male fruit vendor presented with complaints of a lump in the right breast since 7 months. The lump developed insidiously and was associated with pain that led him to seek medical intervention. He did not have any associated medical co-morbidities and was not on any chronic medications. He did not have any history of gynaecomastia and was not on any form of hormone replacement therapy. His scrotum was normal on examination. He did not have any family history of breast or ovarian cancers. On retrospective questioning he admitted that would often consume ranitidine tablets for symptoms of gastritis which was easily available over the counter. On examination he had a 3*3 cm hard lump in the central quadrant of the right breast; the mobility ofwhich was restricted indicating fixity to the pectoralis muscle (Fig. 1). There was another hard 1*1 cm solitary lump lateral but separate from the aforementioned lump. One hard solitary central group lymph node was palpable on examination of the right axilla. Clincal AJCC stage of the tumour was T4cN1M0. Ultra sonogram of the breast and axilla complimented the clinical findings. A whole body PET-CT did not reveal any evidence of distant metastasis. Fine needle cytology evaluation confirmed the diagnosis of carcinoma breast in the primary lump, the satellite nodule as well as in the axillary lymph node. The patient underwent a modified radical mastectomy of the right breast (Fig. 2 and 3). Histopathological examination of the specimen showed invasive malignant tumour comprising of tumour cells arranged in nests and trabeculae. The tumour cells possessed round nuclei with minimal atypia, vesicular chromatin and prominent nucleoli. Stromal desmoplasia was seen along with mild lymphocytic infiltration. Multiple microcalcifications were present along with some perineural involvement. The overlying skin and underlying skeletal muscles were involved. Metastasis was present in 3 of the 9 lymph nodes studied along with extranodal spread. These findings were consistent with invasive lobular carcinoma (Fig. 4 and 5). Pathological AJCC stage was IIIB (pT4N1M0). The tumour was both estrogen and progesterone receptor positive on immunohistochemistry. The patient was advised to receive adjuvant chemotherapy but was not compliant. However, he has been on Tamoxifen (100 mg) for the last four years and has had no recurrence.
DISCUSSION
Breast carcinoma in men is an exceedingly rare entity. The prevalence of which is one per 100,000 persons and accounts for less than 1% of all breast cancers. It is responsible for less than 1% of all male malignancies [1]. The average age at diagnosis is usually between 63 and 71 years which is five years older compared to women with a range of 58 to 93 years [1, 2, 3]. This gentleman was 75 years old at the time of diagnosis which is a little more than the average, but consistent with the fact that male breast cancers occur in the 7th and 8th decades of life. Since male breast carcinomas have a low rate of incidence the exact aetiology remains largely unknown. However, some risk factors have been elucidated. Androgen aromatisation, obesity and alcoholic liver disease all of which are associated with increased levels of sex steroid binding globulin. These in turn incite a state of oestrogen excess and remain the most probable aetiologies [4]. Undescended testes, orchitis, orchiectomy, and sterility are other risk factors, albeit a causal association is yet to be determined. Some medications, such as cimetidine, are thought to multiply the risk of male breast cancer due to hyperestrogenism [5, 6]. A few controlled studies in animals and man have shown no stimulation of any pituitary hormone by ranitidine hydrochloride and no antiandrogenic activity. Studies have even claimed that cimetidine-induced gynaecomastia and impotence reduced when cimetidine was substituted with ranitidine [7]. However our patient was on long term over the counter ranitidine treatment. Whether ranitidine also has the same properties of its predecessor cimetidine remains unknown. Anecdotal evidence such as this may not be sufficient to justify our claim, but there certainly is a possibility that should be looked at. Men who have a family history of female breast cancer are at 2.5 times increases risk when compared to those with no family history [8]. Some studies attribute mutations in the genes BRCA1 and BRCA2 to male breast cancer but with a lower absolute risk and frequency compared to women [9]. Data from SEER (Surveillance, Epidemiology, and End Results cancer registry) of 2000 patients showed that 93.7% of breast cancers are ductal man or unclassified, 2.6% were papillary, 1.8% is mucinous and only 1.5% are lobular [2]. The ductal carcinoma in situ (DCIS) accounts for 10% of male breast cancers [2]. The in situ lobular carcinoma (LCIS) is rare due to the absence of terminal lobules, although it is found in association with ILC [10]. Goss et al. reported 1.9% of all male breast cancers as ILC [11]. Nahleh et al. reported the highest percentage of ILC when they analysed the Veterans Affairs Central Cancer registry with 612 male breast cancer patients [12]. ILC is completely different entity from the much more common invasive ductal carcinoma (IDC). Although ILC accounts for 12% of all female breast cancers, they are exceedingly rare in the male breast [13]. Lobular histologic subtype of breast carcinoma is due to the lack of acini and lobules in the normal male breast [14]. Endogenous or exogenous estrogenic stimulation may incite the development of acini and lobules in the male breast; this is referred as ‘lobulization’of the male breast. This could theoretically increase the risk of development of ILC in males [14]. ILC displays a wide variety of histologic subtypes from classical through solid to pleomorphic forms. The classical variant is found to have better prognosis. Male breast cancers are strongly hormone dependent tumours. Almost 90 and 81% are estrogen and progesterone receptor positive respectively [2, 15]. But overexpression and/or amplification of the Her2 gene is less than IDC [16]. Our patient was both oestrogen and progesterone receptor positive and has been on Tamoxifen daily for the last 4 years.
ILC characteristically lacks E-cadherin expression on immunohistochemical (IHC) staining and this property is now used as a diagnostic aid [10]. We were unable to carry out IHC staining due to cost constraints and hence reserve any judgements. Male breast cancer carries a worse prognosis than female [2]. This could be attributed to the lack the lobular component allowing the tumour to invade local structures like the chest wall more rapidly and also distant spread occurs sooner due to the same reason. Tumour size and the lymph node involvement are important prognostic factors just like in females [2]. Men with T2 tumours have a 40% increased mortality risk compared to those with T1 tumours [5]. Our patient presented with a T4a tumour for which he underwent a modified radical mastectomy. Despite having chosen not to receive adjuvant chemotherapy he has no evidence of recurrence and has survived for 4 years after the surgery. We do not wish to enter the debate on the need for adjuvant chemotherapy as the evidence is only anecdotal.
CONCLUSIONS
• The first case of ILC without known estrogen exposure was reported in 1989 [13], and about 30 cases have been reported since. Hence we felt the need to report this case.
• This gentleman had no history of gynaecomastia, no evidence of testicular abnormalities, no liver cirrhosis, no family history of breast or ovarian cancer, and was not on any hormone replacement therapy, but still developed invasive lobular breast carcinoma.
• The only possible contributory factor was chronic consumption of ranitidine.
• Ranitidine is easily available over the counter at a fraction of the cost compared to proton pump inhibitors. Dyspeptic symptoms are easily attributed to gastritis, especially in the elderly.
• Although there is isn’t sufficient evidence to suggest that ranitidine is a potential risk factor, there lies a pressing need to study this association further.
References:
1. Tunon de Lara C, G Goudy, MacGrogan G, M Durand, JM Dilhuydy, Avril A, E Stoeckle, I Bussieres, Debled M, De Mascarel I, Mauriac L. Breast cancer in humans: about 52 If supported in BergoniéInstitute of Bordeaux between 1980 and 2004. Obstetrics and Gynecology Fertility 2008 Apr; 36 (4): 386-394.
2. Giordano SH, DS Cohen, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004 Jul 1; 101 (1): 51-7.
3. Goss PE, Reid C, Pintilie M, Lim R, N Miller. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital Pendant 40 years: 1955-1996 Cancer. 1999 Feb 1; 85 (3): 629-39.
4. JR Weiss, Moysich KB, Swede H. Epidemiology of male breast cancer. Cancer Epidemiol . Biomarkers Prev 2005; 14 (1): 20- 26.
5. Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of breast cancer in men with liver cirrhosis. Am J Gastroenterol. 1998;93:231–233.
6. San Miguel P, Sancho M, Enriquez JL, Fernandez J, GonzalezPalacios F. Lobular carcinoma of the male breast associated with the use of cimetidine. Virchows Arch. 1997;430: 261–263.
7. The Internet Drug Index (RxList). California: The Internet Drug Index [Cited 2015 March 1]. Available from: http://www.rxlist. com/zantac-drug/side-effects-interactions.htm
8. Laabadi K, Jayi S, Alaoui FF, et al. Breast Cancer man: about 6 cases. The Pan African Medical Journal 2013; 16: 70.
9. Sverdlov RS Barshack I, Bar Sade RB, RG Baruch, Hirsh-G Yehezkel, Dagan E, Feinmesser M Freeze A, Friedman E. Genetic analysis of male breast cancer in Israel. Genet Test. 2000; 4 (3): 313-7.
10. Espiémillion Gorins A. Breast normal to pathological: state of the art. Oxford University Press; 2001.
11. Goss PE, Reid C, Pintilie M, Lim R, Miller N. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955-1996. Cancer. 1999;85:629–639.
12. Nahleh ZA, Srikantiah R, Safa M, Jazieh AR, Muhleman A, Komrokji R. Male breast cancer in the veterans affairs population: a comparative analysis. Cancer. 2007;109: 1471–1477.
13. Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005;10(7):471–479.
14. Zahir MN, Minhas K, Shabbir-Moosajee M. Pleomorphic lobular carcinoma of the male breast with axillary lymph node involvement: a case report and review of literature. BMC Clinical Pathology 2014;14:16.
15. Maly B, Maly A, Pappo I, Meir K, Pappo O. Pleomorphic variant of invasive lobular carcinoma of the male breast. Virchows Arch. 2005;446(3):344–345.
16. Varga Z1, Mallon E. Histology and immunophenotype of invasive lobular breast cancer. Daily practice and pitfalls. Breast Dis. 2008-2009; 30:15-9.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License