IJCRR - 7(17), September, 2015
Pages: 27-33
Date of Publication: 11-Sep-2015
Print Article
Download XML Download PDF
PREVALENCE OF CARDIAC COMORBIDITIES AND ITS RELATION TO SEVERITY STAGING OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Author: Vineeth Alexander, R. Pajanivel, K. Surendra Menon, Arun Prasath
Category: Healthcare
Abstract:Background: Complexity of COPD and mortality from the disease is increased by co morbidities and exacerbations Objective: This study was conducted with aim to find the prevalence of cardiac co morbidity in COPD and its relation to severity staging of COPD. Methods: The present cross sectional study was done in Pulmonary Medicine outpatient department of Mahatma Gandhi Medical College and Research Institute, Pondicherry from March 2013 to June 2014. The study diagnosed and newly diagnosed of COPD patients were subjected to Pulmonary Function Test (PFT), assessment of blood pressure, electrocardiography and echocardiography. The statistical analysis was done to assess the cardiovascular status of the study subjects and its relation to severity staging of COPD. Results: In the total of 44 cases selected for study 42 (95.5%) were males and 2(4.5%) were females. On the basis of GOLD guidelines there were 5(11.4%), 13(29.5%), 16(36.4%) and 10(22.7%) mild, moderate, severe, and very severe COPD respectively. Right axis deviation, p-pulmonale, T-wave inversions, dominant R-wave, persistent S-wave in electrocardiography were present in 45.5%,52.6%,40.0%,33.3%,36.4% of severe and 54.5%, 36.8%, 60.0%, 58.3%, 63.6% in very severe cases of COPD. In echocardiography, right atrium and ventricle dilatation, left ventricular dysfunction, tricuspid regurgitation, and regional wall motion abnormalities were present in 55.6%, 46.15%, 50.0%, 37.5% of severe and 38.9%, 53.85%, 33.3%, 62.5% of very severe cases of COPD. Pulmonary artery systolic pressure and systemic hypertension increased with severity of COPD.
Conclusion: Prevalence of cardiac co morbidities increases with the increase in severity of COPD. The severe and very severe stages of COPD are associated with significant cardiovascular diseases.
Keywords: COPD, Electrocardiography, Echocardiography, Pulmonary artery systolic pressure
Full Text:
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow limitation that is not fully reversible1 . It is a leading cause of death worldwide2 . COPD bring forth high healthcare costs3 , imposes a significant burden in footing of disability and impaired quality of life4 . It is an important public health dispute that is both preventable and treatable. Among the diseases causing chronic morbidity and mortality throughout the world many elderly people suffer from COPD and die prematurely from it or its complications. In coming decades because of continued exposure to risk factors, COPD is projected to increase globally5 . COPD has been considered symptomatically as chronic bronchitis, anatomically as emphysema, physiologically as airflow obstruction in the past6 . Both genetic and environmental factors play a role in development of COPD. In addition to tobacco smoke, heavy exposure to occupational dusts, chemicals and indoor/outdoor air pollution may cause
COPD. The hereditary deficiency of 1-antitrypsin is genetic risk factor for development of COPD7-9. The socioeconomic status is inversely related to the development of COPD10. Complexity and mortality of COPD is increased by its co morbidities and exacerbations11-14. COPD is a more complex systemic disease that has significant extra pulmonary effects along with pulmonary involvement15-17. In relation to COPD and its manifestations and co morbidities there are two different views. The first view is that there is systemic spillover of the inflammatory and reparatory events occurring in the lungs of COPD patients and second view is that the pulmonary manifestations occurring in COPD is just a form of expression of systemic inflammatory state with multiple organ compromise14,18. In two-thirds of the COPD patients there is one or two co morbidities19. The most common co morbidities described in association with COPD are arterial hypertension, coronary artery disease, heart failure, respiratory infections, lung cancer, diabetes mellitus and osteoporosis. There is significant impact of co morbidities on health status, healthcare costs and prognosis of COPD. The mortality is more from co morbid disease than COPD itself20-22. The most frequent and most important disease coexisting with COPD is cardiovascular diseases23, 24. Cardiovascular diseases particularly ischemic heart disease has been observed as the cause of death in COPD patients recently25, 26. It may be associated with smoking as it is a cause of both. But forced expiratory volume in first second (FEV1) is a well known risk factor for the development of ischemic heart disease that is independent of smoking habit27. There is limited studies and research into the co-morbidities of COPD. Most of the studies have analyzed the relation of COPD with several isolated diseases. This study was conducted to find the prevalence of cardiac co-morbidity in relation to severity staging of COPD.
METHODOLOGY
This study was an institutional cross sectional study done in Pulmonary Medicine outpatient department of Mahatma Gandhi Medical College and Research Institute, Pondicherry from March 2013 to June 2014. The study was approved by the institutional ethical committee. The study subjects were all the patients who were previously diagnosed and newly diagnosed of COPD attending the Pulmonary Medicine outpatient department of Mahatma Gandhi Medical College and Research Institute, Pondicherry selected by series allocation from March 2013 to June 2014. All patients with acute exacerbation of COPD unable to perform spirometry and patients with contraindication for spirometry like history of recent myocardial infarction, congenital heart disease were excluded from the study. A volunteer written consent was taken from all the patients before the study. The selected patients were subjected to Pulmonary Function Test (PFT) with flow sensing PFT machine of MIR Spirobank 2 and assessed for severity and stage of COPD according to GOLD guidelines as per follows.
Classification of Severity of Airflow Limitation in COPD according to GOLD guidelines
Based on Post Bronchodilator Forced Expiratory Volume in first second (FEV1).In patients with FEV1/Forced Vital Capacity (FVC) < 0.70:

In the established cases of COPD and the newly diagnosed cases of COPD, blood pressure assessment, electrocardiography (ECG) and transthoracic Doppler echocardiography was done to assess for cardiac abnormalities. Blood pressure was measured using a calibrated sphygmomanometer. The study was done by taking two independent blood pressure measurements with 5 min pause after a rest of 5 min in a sitting position. In current analysis the mean of two measurements was taken. The blood pressure was classified according to the guidelines of Seventh Joint National Committee (JNC 7).

All the patients were subjected to Electrocardiography (ECG) using machine of Mortara ELI 250. A twelve lead ECG including 3 bipolar limb leads, 3 unipolar limb leads and 6 unipolar precordial leads was performed. All necessary precautions desired in ECG were observed. Various ECG parameters like rate, axis deviation, P-wave changes, QRS complex, T-wave changes, ST changes were observed. The axis of P-value and QRS complex was calculated by hexaxial reference system. All patients were then subjected to transthoracic Doppler echocardiography using machine of Philips iE33 with a multi frequency probe of 2- 4.3 MHz to assess for, right side chamber size, left ventricle function, valvular status and pulmonary artery systolic function according to American Society for Echocardiography (ASE) guidelines. Tricuspid regurgitant flow was identified by color flow Doppler technique and the maximum jet velocity was measured by continuous wave Doppler without the use of intravenous contrast. In the absence of right ventricular outflow obstruction the pulmonary artery systolic pressure equals the right ventricular systolic pressure (RVSP) in echocardiography. The Modified Bernoulli equation (?p=4V2) was used, where ?p is the pressure gradient between the right ventricle and right atrium and v is the velocity of the tricuspid regurgitant jet. Right ventricular systolic pressure was calculated as: right ventricular systolic pressure = 4TRV2 + RAP where v is the velocity of the tricuspid regurgitant jet and RAP the right atrial pressure. Right atrial pressure was estimated from the inferior vena cava imaged with two-dimensional echocardiography. RAP was estimated to be 5, 10, or 15 mmHg based on the variation in the size of inferior vena cava with inspiration as follows: complete collapse, RAP = 5 mmHg; partial collapse, RAP = 10 mmHg; and no collapse, RAP = 15 mmHg28. Pulmonary hypertension (PH) was defined in this study as Pulmonary Artery Systolic Pressure (PASP) ≥ 30 mmHg28. This value was chosen according to the definition of pulmonary hypertension. Pulmonary hypertension was classified into mild, moderate, and severe category as PASP 30–50, 50–70, >70 mmHg, respectively.
STATISICAL METHOD
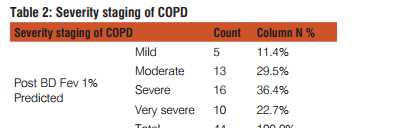
SPSS version 19.0 (IBM SPSS, US) was used to analyze the data. The quantitative variables have been described as mean ± SD or Frequency analysis with numbers and percentage. The study was statistically analyzed by Pearson Chi- square test. Value of p< 0.05 was considered significant. RESULTS In a total of 44 patients with COPD enrolled in the study 42(95.5%) were males and 2(4.5%) were females. The minimum age observed was 42 years and maximum 79 years. The mean age group in our study was 61.25 with a standard deviation of 8.662. The distribution of patients with severity staging of COPD on the basis of GOLD guidelines described in Table 2
The ECG changes observed in our study were right axis deviation, p pulmonale, t wave inversion in V1 and V2 was dominant r wave in V1 lead, persistent S wave in V5 and V6. The changes of right axis deviation was seen in 11(25.0%) of which 5(45.5%) and 6(54.5%) was severe and very severe COPD respectively (p value 0.005). The presence of P pulmonale was observed in 19(43.2%) patients of which 2(10.5%), 10(52.6%), 7(36.8%) were moderate, severe and very severe COPD respectively (p value 0.004). The changes of T wave inversion in V1 and V2 lead was observed in 5(11.4%) of which 2(40.0%) and 3(60.0%) was severe and very severe COPD respectively. The changes of dominant R wave in V1 lead was seen in 12(27.3%) of which 1(8.3%), 4(33.3%) and 7(58.3%) was moderate, severe and very severe COPD respectively (p value0.003). The changes of persistent S wave in V5 and V6 lead seen in 11(25.0%) of which 4(36.4%) and 7(63.6%) was severe and very severe COPD respectively (p value 0.001). The echocardiography findings seen in our study were right atrium and ventricle dilatation, left ventricular dysfunction, tricuspid regurgitation, regional wall motion abnormality and increase in Pulmonary artery systolic pressure (PASP). The changes of right atrium and ventricle dilatation was seen in 18(40.9%) of which 1(5.6%), 10(55.6%) and 7(38.9%) was moderate, severe and very severe COPD respectively (p value 0.001). There was left ventricular dysfunction in 13(29.5%) of which 6(46.15%) and 7(53.85%) was severe and very severe COPD respectively (p value 0.001). The changes of tricuspid regurgitation was seen in 24(54.5%) of which 4(16.7%), 12(50.0%) and 8(33.3%) was moderate, severe and very severe COPD respectively (p value 0.003). The presence of regional wall motion abnormality was seen in 8(18.2%) of which 3(37.5%) and 5(62.5%) was severe and very severe COPD respectively (p value 0.013). The Pulmonary artery systolic pressure (PASP) observed were mild (30-50mmHg), moderate (50-70 mmHg) and severe (>70 mmHg) in 26(59.09%), 13(29.55%) and 5(11.36%) of patients respectively. The distribution of PASP on the basis of severity staging of COPD is depicted in Figure 1

The assessment of blood pressure in our study showed distribution in all stages as depicted in Figure 2.

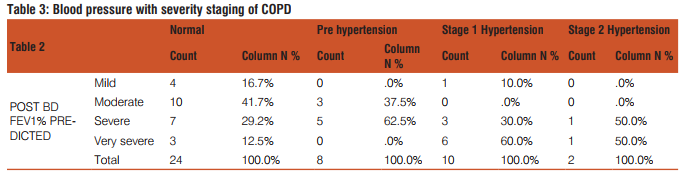
The correlation of blood pressure with severity staging of COPD is depicted in Table3.

DISCUSSION
There are various cardiac manifestations in COPD which complicate its clinical course. In patients with COPD with associated cardiovascular diseases the morbidity and mortality is seen to be increased as shown in various studies29-31. COPD and cardiovascular diseases has various common risk factors, including smoking and aging. The presence of pro inflammatory mechanism and oxidative stress is seen in both diseases32-35. The sedentary lifestyle in COPD may also contribute to risk of developing cardiovascular diseases36. Among the 44 cases in our study all patients had ECG changes. The changes of right axis deviation in ECG were present in only severe and very severe COPD in our study. In a study by Padmavati et al the observation of right axis deviation in ECG of COPD patients were found to be 80%37. In concordance with our results, a study by D Holtzman et al reported high prevalence of right axis deviation inECG in COPD patients, increasing with severity of the disease38. P pulmonale is diagnosed when the amplitude of P wave in Lead II, III, and/or aVF is more than 2.5 mm. In a study by D.H Spodicks et al39, p pulmonale was observed in 13.9% of COPD patients. F. I Carid et al40 found the incidence of p pulmonale in 15.5% while R.C Scott et al41 and Pinto et al42 done a study on COPD patients showing the incidence of p pulmonale of 32.7%. In an Indian study by Aggarwal et al43 the incidence of p pulmonale was found to be 35.7%. In our study the p pulmonale was observed more in severe COPD 10(52.6%). However in our study the T wave inversion in V1 and V2 leads were not statistically significant but was seen in 2(40.0%) and 3(60.0%) patients of severe and very severe COPD respectively. Our study showed statistically significant changes in ECG like dominant R wave in V1 lead and persistent S wave in V5 and V6 increasing with the severity of COPD. The observed data shows that the features suggesting right ventricular hypertrophy increases with severity of COPD with more number of cases reported in severe and very severe stages of COPD. In our study echocardiography changes of right atrium and ventricle dilatation were seen increasing in severe cases of COPD. Soriano et al23 the overall prevalence of heart failure in COPD was observed as 7%. It was corresponding to the severity of airflow limitation. In a study by Higham M.A et al in which the presence of tricuspid regurgitation (TR) was observed in 56(77%) out of 73 COPD patients44. We also observed a significant number of patients in severe and very severe COPD with this abnormality. Our study showed that a significant number of patients also had regional wall motion abnormality and left ventricular dysfunction. In a study the prevalence of chronic obstructive pulmonary disease in patients with catheter diagnosed coronary artery disease by Ahmed A.H et al has shown that more than 1 in 4 patients with coronary artery disease had concomitant COPD45. In a study by Reed R.M et al prevalence of angiographically proven coronary artery disease in COPD was 59%46. Pulmonary hypertension (PH) was defined in this study as Pulmonary Artery Systolic Pressure (PASP) ≥ 30 mmHg28. This value was chosen according to the definition of pulmonary hypertension. Pulmonary hypertension was classified into mild, moderate, and severe category as PASP 30–50, 50–70, >70 mmHg, respectively. In our study the following was the distribution as depicted in Table4

In another study by M.A Higham et al the pulmonary artery systolic pressure was increased in 25%, 43%, and 68% of patients with mild, moderate, and severe COPD, respectively44. There is evidence suggesting that elevation of pulmonary arterial pressure is reported to occur in twenty to ninety percent of patients of COPD47-50. The presence of Cor pulmonale was seen in approximately 25% patients with COPD51. An autopsy study showed Cor pulmonale in 40% patients with COPD49,52. The Correlation of blood pressure with severity staging of COPD in our study is depicted in Table 5.

In a study by Engstrom et al it was found that lung function was inversely associated with future blood pressure increase53. Our study also showed that the mean distribution of patients with increased blood pressure were more in advance stages of COPD. The study has some limitations. First the sample size was less. Second, the absence of a control group limits a definite assessment of the role of COPD in the pathogenesis of cardiac disorders. Thirdly, the study had a cross-sectional design, so no causal relationships with clinical outcomes could be established. Studies with larger sample size with a longer duration will be required to assess the outcome. The other co morbidities in COPD should have to be taken for study with considering the individual as whole. The study indicates that COPD is associated with a higher risk for cardiovascular diseases and the risk of cardiovascular diseases increases with the severity of COPD.
CONCLUSION
The study showed that cardiac disorders are highly prevalent in patients with severe-to-very severe COPD. All COPD patients must be evaluated for cardiac co- morbidities, since it might help establish adequate treatment that may potentially improve patient prognosis.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to author/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors also acknowledge all the teaching and non teaching faculties and fellow postgraduates of Mahatma
Gandhi Medical College, Pondicherry who have helped in the completion of this study.
Source of funding- No funding received
Conflict of interest- The authors do not have any conflicts of interest to disclose.
References:
1. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS (2001) GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Resp Crit Care Med 163:1256 – 1276.
2. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet 2003; 362: 1053–1061.
3. Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000; 117: 5S–9S.
4. Ferrer M, Alonso J, Morera J, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. Ann Intern Med 1997; 127: 1072–1079.
5. Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412.
6. Snider GL. Nosology for our day: its application to chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167: 678–683.
7. Laurell CB, Eriksson S. The electrophoretic alpha-1 globulin pattern of serum in alpha-1 antitrypsin deficiency. Scand J Clin Lab Invest 1963;15:132–140.
8. Hubbard RC, Crystal RG. Antiproteases. In: RB Crystal, JB West, PJ Barnes, NS Cherniack, ER Weibel, editors. The lung: scientific foun- dations. New York: Raven Press; 1991. p. 1775– 1787.
9. McElvaney NG, Crystal RG. Inherited susceptibility of the lung to pro- teolytic injury. In: RG Crystal, JB West, ER Weibel, PJ Barnes, edi- tors. The Lung: Scientific Foundations, 2nd ed. Philadelphia: Lippin- cott-Raven; 1997. p. 2537–2553.
10. Prescott E, Lange P, Vestbo J. Socioeconomic status, lung function and admission to hospital for COPD: results from the Copenhagen City Heart Study. Eur Respir J 1999;13:1109–1114.
11. GOLD - the Global initiative for chronic Obstructive Lung Disease [updated 2013 Feb 1; accessed 2013 Jun 20]. Available from: http://www. goldcopd.org/guidelines-global-strategy-fordiagnosis-management.html.
12. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962–969.
13. Soler-Catalun ?a JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60: 925–931.
14. Sevenoaks MJ, Stockley RA. Chronic obstructive pul- monary disease, inflammation and co-morbidity – a common inflammatory phenotype? Respir Res 2006; 7: 70.
15. Nussbaumer-Ochner Y, Rabe KF. Systemic manifestations of COPD. Chest. 2011;139:165---73.
16. Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:549---55.
17. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165---85.
18. Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 2007; 370: 797–799.
19. Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18:213---21.
20. McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascer- tainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411---5.
21. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233---9.
22. Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245---57.
23. Soriano JB, V isick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005;128:2099-107.
24. Fabbri LM, Luppi F , Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J 2008;31:204-12.
25. Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J. 2003;22:809-14.
26. Huiart L, Erns P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640-6.
27. Hole J, Watt GC, Davey-Smith, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711-5.
28. Rappaport E. Cor pulmonale. In: Murray JJ, Nadel JA, Mason RM, Boushey H, editors. Textbook of respiratory medicine. 4th Edition. Philadelphia: W.B. Saunders; 2000. pp. 1631–48.
29. Dankner R, Goldbourt U, Boyko V, et al. Predictors of cardiac and noncardiac mortality among 14,097 patients with coronary heart disease: BIP Study Group. Am J Cardiol 2003; 91:121- 127.
30. Behar S, Panosh A, Reicher-Reiss H, et al. Prevalence and prognosis of chronic obstructive pulmonary disease among 5,839 consecutive patients with acute myocardial infarction: SPRINT Study Group. Am J Med 1992; 93:637-64.
31. Samuels LE, Kaufman MS, Morris RJ, et al. Coronary artery bypass grafting in patients with COPD. Chest 1998; 113:878-882.
32. Agustí A , Edwards LD , Rennard SI , et al ; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators . Persistent systemic infl ammation is associated with poor clinical outcomes in COPD: a novel phenotype . PLoS ONE . 2012 ; 7 ( 5 ): e37483 .
33. Agustí AG , Noguera A , Sauleda J , Sala E , Pons J , Busquets X . Systemic effects of chronic obstructive pulmonary disease . Eur Respir J . 2003 ; 21 ( 2 ): 347 - 360 .
34. Gan WQ , Man SF , Senthilselvan A , Sin DD . Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis .Thorax2004 ; 59 ( 7 ): 574 - 580.
35. MacNee W . Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease . Proc Am Thorac Soc. 2005 ;2( 1 ):50 - 60 .
36. Garcia-Aymerich J , Lange P , Benet M , Schnohr P , Antó JM . Regular physical activity modifi es smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study . Am J Respir Crit Care Med. 2007 ; 175 (5): 458 - 463.
37. Padmavati S, Raizada V. Electrocardiogram in chronic cor pul-monale. Br Heart J. 1972 Jul;34(7):658–667.
38. Holtzman D, Aronow WS, Mellana WM, Sharma M, Mehta N, Lim J, et al. Electrocardiographic abnormalities in patients with severe versus mild or moderate chronic obstructive pulmonary disease followed in an academic outpatient pulmonary clinic. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc. 2011 Jan;16(1):30–2.
39. Spodick DH, Hauger - Kelvene JH, Tyler JM, Muesch H, Dorr CA. The electrocardiogram in pulmonary emphysema. Relationship of characteristic electrocardiographic findings to severity of disease as measured by degree of airway obstruction. Am Rev Resp Dis 1963; 88:14.
40. Carid FI and Wilcken DEL. ECG in chronic bronchitis with generalised obstructive lung diseases - Its relation to ventilatory junction. Am J Card 1962; 10:5.
41. Scott RC, Kaplan S, Fowler O, Helm RA, Westcott RN, Walker IC et al. The electrocardiographic pattern of right ventricular hypertrophy in chronic corpulmonale Circulation 1955; 11:927. 42. Pinto, Hansoti RC. The ECG changes in chronic corpulmonale. J Assoc Phy India 1960; 8:213.
43. Agarwal, R; Kumar, Dinesh; Gurpreet; Agarwal, D; Chabra, G.Diagnostic values of electrocardiogram in chronic obstructive pulmonary disease (COPD) Lung India25.2 (Apr 2008): 78-81.
44. Higham MA, Dawson D, Joshi J, Nihoyannopoulos P, Morrell NW. Utility of echocardiography in assessment of pulmonary hypertension secondary to COPD. Eur Respir J. 2001;17:350–5.
45. Ahmed AH, Yagoub TE, Muthana F. Prevalence of chronic obstructive pulmonary disease in patients with catheter-diagnosed coronary artery disease. Ann Thorac Med. 2009 Apr;4(2):91–2.
46. Reed RM, Eberlein M, Girgis RE, Hashmi S, Iacono A, Jones S, et al. Coronary artery disease is under-diagnosed and under-treated in advanced lung disease. Am J Med. 2012 Dec;125(12):1228. e13–1228.e22.
47. Weitzenblum E, Hirth C, Ducolone A, Mirhom R, Rasaholinjanahary J, Ehrhart M. Prognostic value of pulmonary artery pressure In chronic COPD. Thorax. 1981;36:752–8.
48. Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener CF. Patterns of cardiovascular dysfunction in COPD. N Engl J Med. 1972;286:912–8.
49. Fishman AP. State of the art: Chronic cor pulmonale. Am Rev Respir Dis. 1976;114:775–94.
50. Thabut G, Dauriat G, Stern JB, Logeart D, Lévy A, MarrashChahla R, et al. Pulmonary haemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–6.
51. Springhouse. Respiratory disorders. In, Springhouse (ed). Professional Guide to Diseases.9th Edition. Philadelphia, Lippincott William and Wilkins 2008;120.
52. Rigolin VH, Robiolio PA, Wilson JS, Harrison JK, Bashore TM. The forgotten chamber: The importance of the right ventricle. Cathet Cardiovasc Diagn 1995;35:18-28.
53. Engström G, Hedblad B, Valind S, Janzon L. Increased incidence of myocardial infarction and stroke in hypertensive men with reduced lung function. J Hyperten 2001;19:295-301.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License