IJCRR - 14(13), July, 2022
Pages: 60-64
Date of Publication: 05-Jul-2022
Print Article
Download XML Download PDF
In-Vitro Antioxidant Activites of Novel Synthesized Copper (II) Complexes
Author: Muhammad Tahir, Muhammad Shahab Khan, Muhammad Bilal, Ashfaq Ahmad, Mukhtar Ahmad
Category: Healthcare
Abstract:Introduction: The term radical is originally implied, to a portion of a molecule that cannot exist independently in nature. The Development of these radicals is driven by thermal hemolysis, high-energy radiation and photolysis. Free radical attacks cause cell damage and homeostatic disruption. Targets of free radicals are all kinds of molecules in the body, such as lipids, nucleic acids and proteins are the major targets. Antioxidants are substances capable of slowing or stopping oxidation pathways that occur under the effect of reactive oxygen moieties or free atmospheric oxygen. Objectives: The main objective of the present study was to judge the advantages of the newly synthesized Copper (II) complexes in contrast to oxidative stress caused by free radicals utilizing different assays. Methods: The antioxidant ability of Cu (II) complexes were measured employing different assays including, DPPH free radical scavenging ability, ferric reducing antioxidant potential (FRAF) and total antioxidant ability assay. Results: In the DPPH scavenging potential assay, ferric reducing antioxidant potential and total antioxidant ability assay, RI-1 complex was studied to be more spectacular among the copper(II) complexes. Inhibiting ability of the complexes was RI-1 > NM-3 > AM-2 > SR-3. The Antioxidant scavenging potential was conformed as dose-dependent which increases with the increase of complex concentration. Conclusion: The Copper (II) complexes were found to have high antioxidant abilities.
Keywords: Antioxidants, Cu (II) complexes, DPPH, Free radicals, FRAF, Total antioxidant ability assay
Full Text:
INTRODUCTION
The current development in the formation of free radicals, reactive Nitrogen species (RNS) and reactive oxygen species (ROS) is producing a medical revolution in science. Antioxidants and free radicals have become usually a main term in modern discussions of disease mechanisms.1 Numerous radical lacking stability and are profoundly reactive in nature. They either give an electron or accept an electron from other molecules.2 These are highly reactive species, fit into the nucleus and in the layers of the cells harming biologically significant molecules such as DNA, proteins, carbohydrates and lipids.3 Free radical reactions can bring irreversible deleterious changes in individuals during their lifetime. These problems can appear in the form of hereditary and environmental changes.4 These seemed as infections, in particular stages induced by hereditary and ecological elements. Cancer and atherosclerosis are two main reasons of death, are remarkable “free radical” infections disorders. It is probable that endogenous radical responses, similar to that started by ionizing radiations may bring about tumor formation.5 Studies on atherosclerosis find out the like hood that the disorders may be because of the free radical’s responses, containing diet-determined lipids in the blood vessels and serum to produce peroxides and different other compounds. These compounds incite endothelial cell damage and bring alterations in the arterial walls.6
Many scientific observations give proves that RNA and DNA are exposed to oxidative damage. Especially it has been investigated that DNA is the major target in ageing and cancer.7 Oxidative nucleotide for example glycol and 8-hydroxy-2-deoxyguanosine is found to be increased during oxidative damage to DNA under UV radiation or free radical damage. It has been reported that mitochondrial DNA is highly susceptible to oxidative damage that have part in various diseases and malignancy. It has been recommended that 8-hydroxy-2-deoxyguanosine can be used as a natural manufacturer for oxidative stress.8 All of the higher classes of biomolecules may be attacked by free radicals but lipids are probably the most susceptible. Cell membranes are the best sources of polyunsaturated fats (PUFAS) which are readily attacked by oxidizing radicals and the process is as known as lipid peroxidation, which is more harmful because it is a self-perpetuating chain reaction.9 The peroxyle radicals are the carriers of the chain reactions they can oxidize PUFA compounds and start new chain reaction to initiate lipid hydroperoxide (LOOH) that can breakdown to yet more radical’s species and to a wide range of compounds, especially aldehydes.10
Antioxidants are known to be free radical scavenger, hydrogen donor, electron transporters, peroxide composer singlet oxygen liquor, catalyst blocker and metal chelating specialists.11 Both catalytic and non-enzymatic cancer prevention agents found in intercellular and cellular condition to decrease toxicity. Man-made and natural food antioxidants are used in food, mostly those containing oils and fats are more important because they inhibit oxidation in foods. Phenolic and butylated hydroxytolune (BHT) are the best examples. The food industry and medical industry have broadly used these complexes as antioxidant.12However, high volatility, instability and cancer-causing nature of butylated hydroxytolune and BHA at high temperature have changed the user’s priority from synthetic to natural antioxidants. The value of certain compounds in medical treatment and genomic research is based on the capacity of the compounds to bind or break double-standard DNA. The hydrolytic and oxidative breaking pathways are tangled in the DNA-breaking reactions. Oxidative cleavage of DNA is bringing about by different methodologies.13
The binding potential of target compound with DNA is the major source for making the comparison of cleavage efficiency of the complexes to that of the control.14 Copper is one of the most important microelement in all living forms and also part of many biological enzymes in the last years, many investigations have shown that the Cu amount of antibody and tissues are considerably higher in the cancer of breast, prostate, lung and brain. Moreover, Cu is also related to angiogenesis, which is essential for tumor proliferation, invasion and metathesis.15 Cu has been utilized in olden days in metal base analyses.16 Cu is a bio, essential element and performs a vital role in life processes and its compounds are selecting agents for a growth malignancy cure.17
MATERIALS AND METHODS
Chemicals and reagents
2,2-Diphenyl-1-picrylhydrazyl radical (DPPH), Ascorbic acid, iron (III) chloride, EDTA, Tris buffer, o-phenenthroline, sulfuric acid, sodium phosphate, ammonium molybdate and ethanol purchased from Sigma Aldrich Pakistan at analytical grade.
DPPH Free Radical Scavenging Ability
A stock solution of 85 M DPPH in ethanol and stock solution of 6000 M of test compound is prepared and different concentration of samples is mixed with a fixed amount of 2,2-diphenyl-1-picrylhydrazyl (DPPH) mixture. Absorbance from all tests are measured immediately up to 2 hrs. Vitamin C is used as a standard.18 The results were intimate as percentage inhibition calculated according to the equation given below:
Inhibition (%) =(Ab - Ac/Ab) ×100
(Where Ab = Absorbance of the control and Ac = Absorbance of the sample)
Iron Reducing Power assay
The reducing powers of sample drugs were measured by using the reported method. The complexes of different concentrations were mixed with Iron (III) chloride, phosphate buffer (PH 6.6) and o-phenanthroline. The absorbance was measured at 490 nm. The increase in absorbance showed the reducing power of the compounds. Ethylenediaminetetraacetic acids (EDTA) were used as a positive control and mixture without the drug were used as a blank.19
Reducing the power of the tested compounds was calculated by using the following formula:
Reducing power (%) = (Ac – Ab/ Ac) × 100
(Where, Ab = Absorbance of the blank and Ac = Absorbance of the sample)
The total antioxidant assays of the tested complexes were determined by using the reported method. All compounds in different concentrations were mixed with sodium phosphate, ammonium molybdate and sulfuric acid into Eppendorf tube. All tubes were heated in the thermal block at 95 0C for 1.5 hrs. After cooling the mixtures to normal temperature, the increase in absorbance was calculated at 695 nm using Vitamin-C as standard.20
Reducing ability of the compounds and ascorbic acid was measured by employing the following formula:
Reducing power (%) = (Ab – As/ Ab) ×100
(Where, Ab = Absorbance of the blank and As = Absorbance of the sample)
Graph pad prism version 6 was used to calculate IC50 ± SEM values.
RESULTS
DPPH radical scavenging assay
Percentage inhibition of the DPPH radicals at different concentration of copper(II) complexes are graphically indicated in graph 1. The IC50 along with SEM values for ascorbic acid was found to be 56.48 + 12.52. The IC50 values for the copper(II) complexes RI -1, NM-3,AM-2 and SR-3 were found to be 12.08 + 9.303,38.25+ 6.081,44.045 + 5.215 and 300.25 + 5.182. Among the copper(II) complexes, scavenging potential of the RI -1 complex was studied to be more spectacular, NM-3 complex represents low ability, the complex AM-2 showed least ability and the complex SR-3 was found to be satisfactory. The DPPH scavenging potential was conformed as amount dependent which increases with the increase of complex concentration.
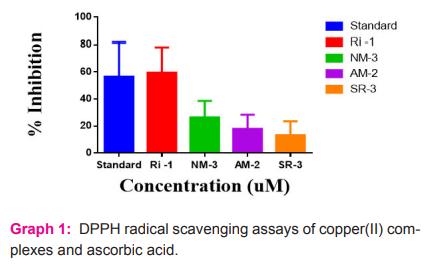
Ferric reducing activity
The copper (II) complexes were evaluated for the antioxidant properties in 0- phenanthroline color method. Graph 2, illustrates the % reduction ±SEM, IC50 ±SEM which shows the significant values of ferric ion indicates the reductive abilities of different evaluating compounds. The IC50 value along with SEM for the ethanolic solution of the standard was found to be 24. 56±8. 544. The IC50 for the copper(II) complexes, RI-1, NM-3, AM-2 and SR-3 were found to be 169. 189±5. 465,121. 075±3. 750,219. 26±3. 180 and 120. 15±11. 16 respectively. Among the copper(II) complexes the compound SR-3 was recorded as high ability to reduce power, NM-3 complex showed less ability, the complex AM-2 and the compound RI -1 was recorded to be satisfactory.
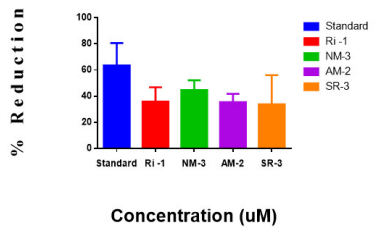
Total Antioxidant capacity
The phosphomolybdate method has been utilized for the evaluation of total antioxidant capacity of the copper(II) complexes using ascorbic acid as a standard. Graph 3, illustrates the % reduction mean at different concentrations with IC50± SEM. The IC50±SEM value of the standard was found to be 61. 74±10. 44. And the IC50±SEM values for the copper(II) complexes RI-1, NM-3, AM-2 and SR-3 were found to be 224. 17±3. 215,422. 26±2. 044,504. 12±1. 412 and 568. 60±2. 622 respectively. Among these compounds RI-1 was found to be better, the compound NM-3 showed less potential, the compound AM-2 and SR-3 were found to be satisfactory.
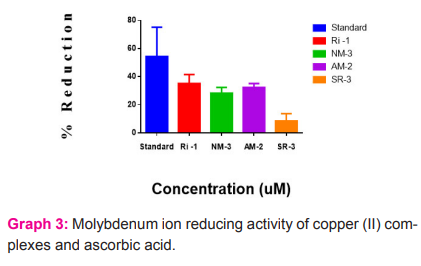
DISCUSSION
DPPH radical scavenging, ferric reducing antioxidant potential (FRAF) and total antioxidant activity assays are widely used techniques for understanding, finding and determining the antioxidant ability of the compounds in view of the responsive capability to find active components at small amount. The absorbance of DPPH radicals is decreased when it interacts with antioxidants. In the present study the DPPH radical scavenging capacity of the copper(II) complexes in ethanol solution was also investigated by using ascorbic acid as a standard. The ferric ion-reducing properties are also dose-dependent and higher activities were recorded at higher concentrations of the compounds comparable with that of standard ascorbic acid. The obtained results present that all compounds in Copper(II) complex actively reduces Mo(VI) ion to Mo(V), Fe(III) ion into Fe(II) ion and also show the reactivity towards DPPH radicals. Of these mechanisms, copper (II) complexes clearly have antioxidant parts that can give an electron or a hydrogen atom. All compounds have a remarkably significant and concentration-dependent antioxidant activity.
Conclusion: The methods used in the current study to evaluate the anti-oxidant properties of copper(II) complexes were well established. Nonetheless, these investigations confirmed the insight into the antioxidant nature of Copper(II) complexes as a beginning of synthetic antioxidants, but due to the complexity and different mechanisms from in vitro, this solution cannot be applied to in vivo system. The research methodologies followed in this work for copper(II) complexes to confirm as a source of synthetic antioxidants are accepted worldwide. In future research, these results should be widened in a mixture of antioxidant methods in vitro and in vivo from the determination of antioxidant content. Research involving other synthetic compounds is also worth investigating in future research.
Abbreviations
BHT: Butylated hydroxytoluene; SEM: Standard error of the mean; ROS: Reactive oxygen species; RNS: Reactive nitrogen species SOD: superoxide DNA: Deoxyribonucleic acid; LOOH: Lipid hydroperoxide; EDTA: Ethylenediaminetetraacetic acid; FRAF: ferric reducing antioxidant potential.
Acknowledgments
The authors are thankful to Higher Education Commission (HEC) of Pakistan. Also, the authors acknowledge Malakand Institute of Medical sciences Dargai, KPK, Pakistan, for providing the facilities for the conduct of the study.
Authors’ contributions
Muhammad Bilal, Ashfaq Ahmadand Mukhtar Ahmad performed the total antioxidant activity assay experiment. Muhammad Tahir and Muhammad Shahab Khan performed the DPPH and FRAF assays experimental work, supervise the whole project and wrote the manuscript. All authors read and approved the manuscript for submission.
Funding
No funding.
Availability of data and materials
The data associated with this study are available from the corresponding author.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no potential competing interest.
References:
1. Bagchi K, Puri S. Free radicals and antioxidants in health and disease: a review. EMHJ-East Mediterr Health J 4 2 350-360 1998. 1998;
2. Chapter 1 Introduction to free radicals. In: Laboratory Techniques in Biochemistry and Molecular Biology. Elsevier; 1991. p. 1–18.
3. Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001 Mar 1;54(3):176–86.
4. Sharma RA, Kumari a. Phytochemistry, pharmacology and therapeutic application of oxalis corniculata linn. - a review. 6(3):7.
5. Lea AJ. Dietary factors associated with death-rates from certain neoplasms in man. Lancet. 1966;2:332–3.
6. Harman D. Role of Free Radicals in Aging and Disease. Ann N Y Acad Sci. 1992;673(1):126–41.
7. Woo RA, McLure KG, Lees-Miller SP, Rancourt DE, Lee PWK. DNA-dependent protein kinase acts upstream of p53 in response to DNA damage. Nature. 1998 Aug;394(6694):700–4.
8. Hattori Y, Nishigori C, Tanaka T, Uchida K, Nikaido O, Osawa T, et al. 8-Hydroxy-2’-Deoxyguanosine Is Increased in Epidermal Cells of Hairless Mice after Chronic Ultraviolet B Exposure. J Invest Dermatol. 1996 Nov;107(5):733–7.
9. Phaniendra A, Jestadi DB, Periyasamy L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J Clin Biochem. 2015 Jan;30(1):11–26.
10. Aruoma I. Peroxyl Radical Scavenging Activity of the Antihypertensive Drug Carvedilol. :5.
11. Ofoedu CE, You L, Osuji CM, Iwouno JO, Kabuo NO, Ojukwu M, et al. Hydrogen Peroxide Effects on Natural-Sourced Polysaccharides: Free Radical Formation/Production, Degradation Process, and Reaction Mechanism—A Critical Synopsis. 2021;33.
12. Zhu F. Triticale: Nutritional composition and food uses. :47.
13. Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009 Jun;59(6):365–78.
14. Schatzschneider U. Photoactivated Biological Activity of Transition?Metal Complexes. Eur J Inorg Chem. 2010;17.
15. Wang M, Wang LF, Li YZ, Li QX, Xu ZD, Qu DM. Antitumor activity of transition metal complexes with the thiosemicarbazone derived from 3-acetylumbelliferone. :4.
16. Akinmoladun AC, Obuotor EM, Farombi EO. Evaluation of Antioxidant and Free Radical Scavenging Capacities of Some Nigerian Indigenous Medicinal Plants. J Med Food. 2010 Apr;13(2):444–51.
17. Puntel RL, Nogueira CW. Krebs Cycle Intermediates Modulate Thiobarbituric Acid Reactive Species (TBARS) Production in Rat Brain In Vitro. :11.
18. Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, et al. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002 Dec;163(6):1161–8.
19. Zhao G, Xiang Z, Ye T, Yuan Y, Guo Z. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem. 2006;99(4):767–74.
20. Kumaran A, Joel KR. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT - Food Sci Technol. 2007 Mar;40(2):344–52.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License