IJCRR - 14(13), July, 2022
Pages: 43-51
Date of Publication: 05-Jul-2022
Print Article
Download XML Download PDF
Respiratory Morbidities and Neurodevelopmental Outcome in Preterm Infants of Mother Exposed to Antenatal Corticosteroids
Author: Mosammad Alpana Jahan, Rabeya Sultana, Tarun Kumar Roy, Sanjoy Kumer Dey, M. A. Mannan, Mohammad Kamrul Hassan Shabuj, Ismat Jahan, Sadeka Choudhury Moni, Mohammod Shahidullah
Category: Healthcare
Abstract:Introduction: Each year, about 15 million babies are born preterm, and 1 million babies die due to complications of preterm birth. Antenatal corticosteroid is one of the most effective evidence-based interventions that can reduce mortality and morbidity in preterm newborns. Objective: The purpose of this study was to evaluate the effect of antenatal corticosteroids on respiratory morbidities and neurodevelopmental outcomes among preterm neonates. Methodology: This prospective cohort study included admitted premature infant's ≤ 34 weeks. Infant's baseline demographics and maternal history of complete ACS exposure or no exposure were evaluated. The development of respiratory morbidities with support was compared between two groups during the neonatal period. After discharge from the NICU, neonates were followed up to assess neurodevelopment at 3rd and 6th months of age using Bayley Scales of Infant Development (BSID-III). Analysis was done to see the relationship with ACS exposure. Results: A total of 82 neonates were enrolled. Among them, 44 neonates in the ACS exposed group and 38 in the ACS unexposed group were assessed for the development of respiratory morbidity. Only 9(20.5%) in the ACS exposed group and 28 (73.7%) in the ACS unexposed group developed respiratory morbidities. Respiratory support with duration was also needed more in the ACS unexposed group. Neurodevelopmental assessment at 3 months was significantly lower in all three domains between the ACS exposed and unexposed groups: cognition (81.67\?7.58 vs. 73.50\?10.16: p-< 0.001), motor (84.50\?9.83 vs. 76.83\?12.03, p-0.013) and language (84.67\?6.33 vs.77.88\?9.98, p-0.004) respectively. At 6 months, motor and language scores improved, though cognition was low in both groups. In univariate analysis, ACS exposure was found to be a significant factor in relation to normal neurodevelopment. Conclusion: Antenatal corticosteroid therapy effectively reduces respiratory morbidities in preterm neonates born ≤ 34 weeks of gestation. ACS exposure was also associated with improved neurodevelopmental outcome.
Keywords: Neonatal period, Neurodevelopmental Outcome, Respiratory morbidity, Antenatal Corticosteroids, Antenatal Corticoster�oid Therapy, Univariate Analysis
Full Text:
Introduction
Under-5 mortality rates are declining in many countries worldwide.But the decreasing trend in newborn mortality has been considerably slower. Currently, prematurity is the leading cause of death among children under five around the world, also a leading cause of disability and ill health later in life. According to the World Health Organization (WHO), about 15 million babies are born preterm each year and about 1 million babies die each year due to complications of preterm birth.1 Bangladesh is one of the top ten countries with the highest number of preterm births in 2010 (424,144 preterm births), around 2.8% of the global preterm burden and 14.1% nationwide.2 Data from the 2017-18 BDHS shows that under-5 mortality in the five years preceding the survey is 45 per 1,000 live births. The infant mortality rate is 38 per 1,000 live births and during infancy, the neonatal mortality rate is 30 per 1,000 live births. Deaths in the neonatal period account for 67 percent of all under-5 deaths.3 Preterm babies may have numerous respiratory complications. The most common respiratory disorders are respiratory distress syndrome, pneumonia and transient tachypnea of the newborn.4 Approximately one-third of preterm survivors also suffer from severe long-term neurological disabilities, such as cerebral palsy or mental retardation.5 Furthermore, preterm infants carry an increased risk of a range of neurodevelopmental impairments and disabilities including behavioral problems, school learning difficulties, and lower growth attainment.6 Early recognition of a delay in neurodevelopment implies that early intervention would have beneficial effects on their development. Antenatal corticosteroid administration has been identified as one of the most important antenatal therapies available to reduce respiratory distress and improve neurological morbidity and mortality in the preterm.ACOG in 2017, 7 and WHO 1 recommended for mothers with gestational age between 24 0/7 weeks and 34 0/7 weeks, 6 mg of Dexamethasone intramuscular (IM) at 12 hours interval for a total of four doses, or 12 mg of Betamethasone intramuscular (IM) at 24 hours interval for a total of two doses, the last dose should be given at least 24 h prior to delivery. This has since then been confirmed in numerous studies. According to the systematic review of 21 studies treatment with ACS is associated with an overall reduction in neonatal death, RDS, IVH, NEC, respiratory support, Intensive care admission and systemic infection in the first 48 hours of life.8 The first randomized control trial of antenatal betamethasone by Liggins and Howie showed reduced mortality rates and respiratory distress syndrome (RDS) in premature infants.9 This antenatal therapy should be considered not only because of the pulmonary maturation but also for its protective role in the premature infant’s brain. Western studies have found antenatal steroid therapy is very effective in preventing neonatal morbidity, but there is little evidence that ACS affects long-term neurodevelopmental and behavioral outcome in 28- to 32-week survivors.10 The use of antenatal corticosteroids has become standard care in cases of imminent or anticipated preterm delivery. In high-income countries, they used antenatal steroid in nearly 90% of cases where indicated, but in low-income countries coverage rates are estimated at 5% in Africa and 9 – 73% in SE Asia 11,12. WHO-recommended antenatal corticosteroids for improving preterm birth outcomes are currently included in Bangladesh’s clinical standards of preterm care at the hospital level. Although the majority of research shows that antenatal corticosteroids protect against respiratory morbidity and mortality, there are scarcity of data in our country that explore the impact of antenatal corticosteroids on respiratory morbidity and neurodevelopmental outcomes.
Materials and methods
This prospective cohort study was conducted in the Department of Neonatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh from July 2018 to June 2019. After taking informed written consent and IRB clearance a total of 82 admitted premature infants with gestational age ≤ 34 weeks whose mother got a complete course of ACS or premature infants whose mother didn’t took any course of ACS were the study population. Hospitalized inborn and outborn babies were included consecutively. Newborn whose mother received an incomplete dose of antenatal corticosteroids or with congenital anomalies, syndromic manifestations or chromosomal malformations and suspected inborn errors of metabolism were excluded from the study. Death within 24 hours of admission in NICU in both ACS exposed and ACS unexposed group were also excluded. Infants’ baseline demographics including gestational age (GA), birth weight (BW), small for gestational age (SGA), gender and perinatal characteristics including place of delivery, mode of delivery, Apgar score at 5 minutes, maternal characteristics including parity, hypertension, diabetes mellitus, presence of risk factors of preterm delivery were collected from history sheet. Birth weight was recorded from labor room or neonatal referral sheet in case of outborn infants. If the neonate was admitted within 6 hours of birth, his/her admission weight was taken as birth weight. Admission weight was taken by a digital weighing scale (SALTER, Model-914). Gestational age was assessed by LMP, sonographic findings and modified new Ballard scoring. In the place of study obstetrician practices inj. Dexamethasone 12.5mg IM 12 hours apart two doses. So in this study, this schedule was taken as a complete dose of ACS. After admission in NICU, neonates were followed up for the development of any respiratory morbidity, if developed relevant investigations and treatment was given according to institutional protocol. Respiratory morbidities were the occurrence of neonatal respiratory disorders: respiratory distress syndrome, pneumonia, TTN or delayed adaptation. Duration with types of support were also analyzed in relation with ACS exposure. After discharge from neonatal unit babies were followed up for neurodevelopmental evaluation at 3 and 6 months of age by appropriately trained clinical psychologists, who were blinded to the child’s neonatal information. Children who attended at least one follow-up study visit were included in the study for analysis. To evaluate development, Bayley Scales of Infant Development (BSID-III), was administered in a calm environment. In the Bayley-III, cognitive development, expressive and receptive language, and fine and gross motor development all were evaluated. Development was classified “normal” if the score is above 85 and “at risk/delayed” if the Bayley III score was below 85 on any of the language, cognitive, or motor scales. Composite scores were analyzed in this study.
Methods of data processing and statistical analyses: All quantitative data were expressed as the mean ± standard deviation. Demographic, perinatal variables, clinical outcome were compared between two groups (ACS exposed and ACS unexposed) using Chi-Squared test for categorical variables and an Independent-t test were applied to calculate the significance in differences of quantitative variables between two groups. Univariate analysis was performed to determine the independent effects of antenatal corticosteroids on neurodevelopmental outcomes. The statistical analyses were performed with the use of SPSS version 20.p-value of equal or less than 0.05 was considered statistically significant.
Results
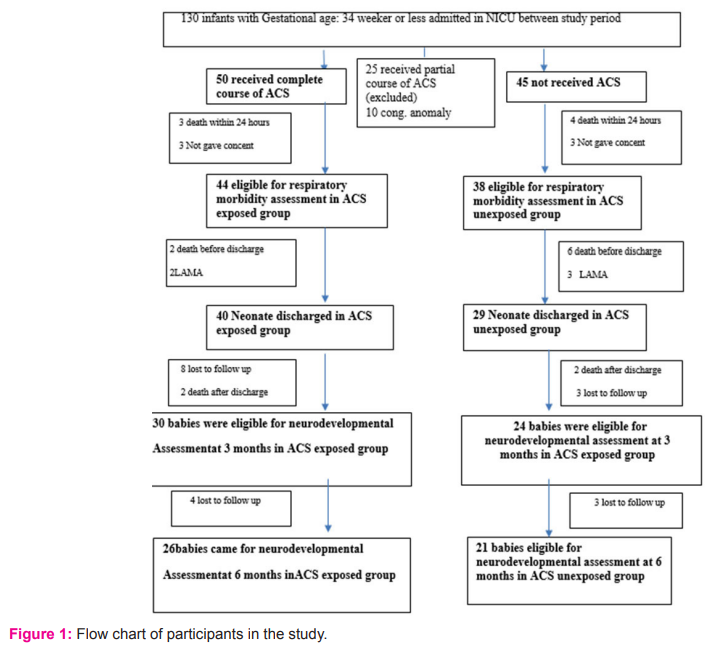
During the study period, 130 eligible preterm infants ≤ 34 weeks gestation were admitted in the Neonatal Intensive Care Unit, BSMMU. A total of 82 neonates were enrolled. Flow chart of participants in the study is given in Fig-1.
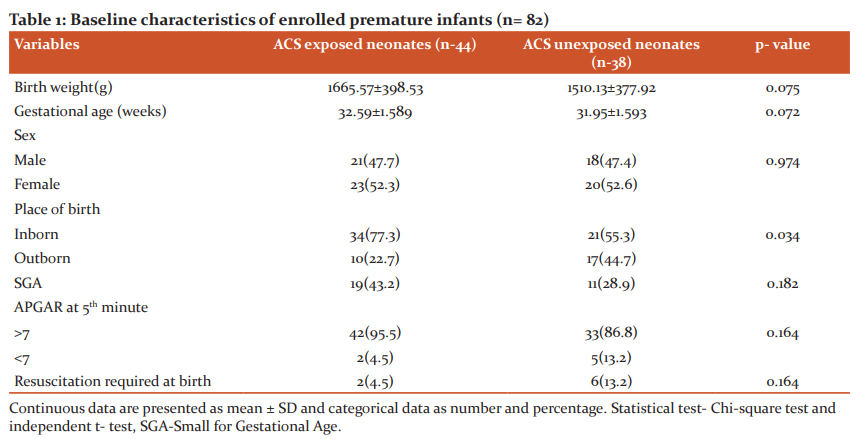
Demographic and baseline characteristics of studied infants were presented in table-1. The ACS exposed and ACS unexposed group had similar baseline characteristics, mean gestational age of ACS exposed and ACS unexposed infants were 32.59±1.58 weeks and 31.95±1.59 weeks, mean birth weight were 1665.57±398.53g and 1510.13±377.92g, respectively. Gender distribution reflects female predominance in both groups (52.3% vs. 47.7% and 52.6% vs 47.4%). It was observed that inborn received more ACS than outborn. Nearly two-third of studied infants was inborn. Neonates who were exposed to ACS were more likely to be small for gestational age.
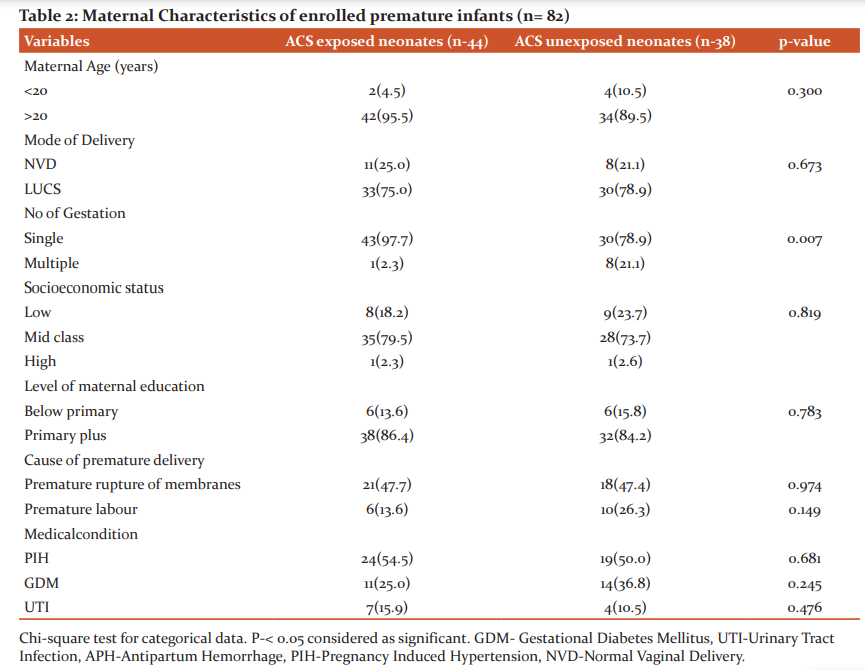
The comparison of maternal data in ACS exposed and ACS unexposed group was presented in Table 2. Cesarean section was the mode of delivery for more than half of the enrolled premature infants. Among the enrolled premature newborns singletons are more in ACS exposed group than ACS unexposed group (97.7% vs. 78.9%, p- 0.007). Maternal hypertension was present 24(54.5%) in ACS exposed infants as compared to 19(50.0%) in ACS unexposed group, however the difference was not statistically significant (p=0.681). In the distributions of the main reason women were considered at risk of preterm birth between the mothers who received corticosteroids and those who did not receive them, more women who received antenatal corticosteroids had premature rupture of membranes (47.7%).
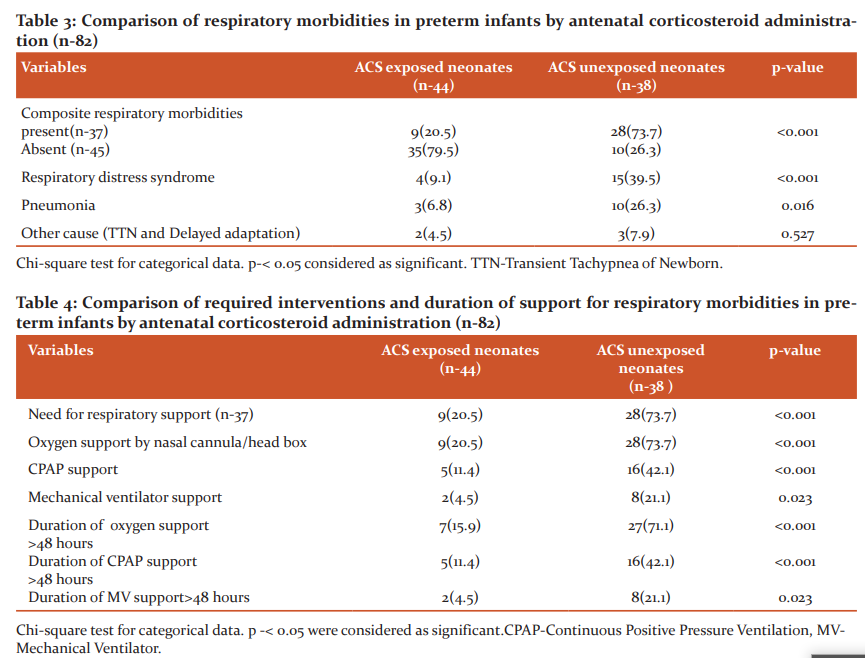
Among 82 enrolled neonates, 37 neonates developed respiratory morbidities (Table-3). Of them 9 neonates were exposed to ACS and 28 were unexposed (p-<0.001). Respiratory distress syndrome was more in ACS unexposed group than ACS exposed group (39.5% vs 9.1%; p-0.001).
Table-4 showing types of respiratory support along with duration in ACS exposed and ACS unexposed group. In ACS unexposed group, respiratory support requirement with duration was more compared to ACS exposed group. All form of supports including nasal cannula, CPAP and Mechanical Ventilator support were needed more in ACS unexposed group which were statistically significant. Duration of support was also needed more in ACS unexposed group.
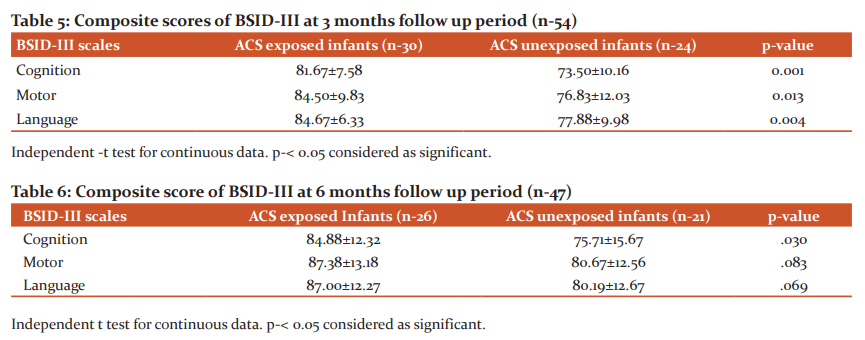
BSID-III at 3 months of age was done among 54 infants showing mean cognitive score were 81.67±7.58 and 73.50±10.16 in ACS-exposed and ACS-unexposed infants (p-0.001).Mean language score in ACS exposed and ACS unexposed group were 84.67±6.33 vs. 77.88±9.98, (p- 0.004) and Mean motor score were 84.50±9.83 vs. 76.83±12.03 (p- 0.013) respectively. Though all scores were below normal in both groups, ACS exposed group had relatively better score than ACS unexposed group at 3 months of age. (Table-5).
During 2nd follow up 47 infants were assessed at 6 months of age.In ACS exposed and unexposed groups mean cognitive score was 84.88±12.32 and 75.71±15.67 which was statistically significant (p-0.030).Motor (87.38±13.18vs80.67±12.56) and language (87.00±12.27vs80.19±12.67) composite scores were higher at 6 months in the ACS group but values were no statistically significant between two groups (Table-6).
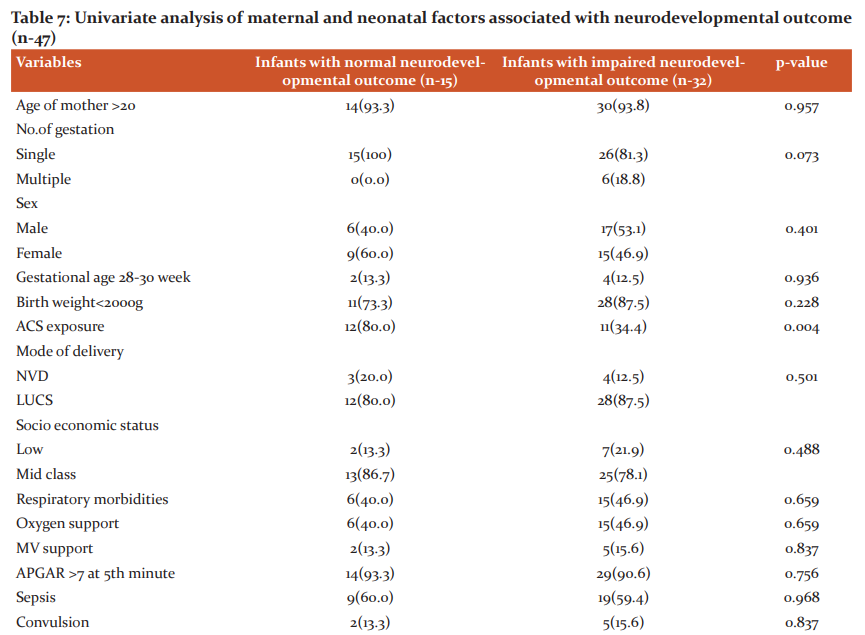
To see the factors associated with adverse neurodevelopmental outcomes, univariate analysis was performed (Table-7). Only ACS exposure was found to be significantly associated as protective factor for normal neurodevelopment (p-0.004).
Discussion
Over the last few decades, the limit of fetal viability has been decreasing due to great advances in obstetrical and neonatal intensive care. As a result, the incidence of preterm infants and their survival is increasing. Administration of antenatal corticosteroid has been found the most effective, safest and least expensive interventions to reduce preterm death. But in developing country like Bangladesh the use of antenatal corticosteroids and its outcome lacks evidence. Thus the aim of this study was to determine the role of antenatal corticosteroids in respiratory morbidities and neurodevelopmental outcome in premature infants. This prospective cohort study was conducted in the department of neonatology, BSMMU illustrates that exposure to antenatal corticosteroids is associated with lower respiratory morbidity and relatively beneficial effect on neurodevelopmental outcome in infants at gestational ages 34 weeks or less, even after adjustment for multiple significant confounders. This study included 82 premature infants with gestational age ≤ 34 weeks, with history of maternal exposure of ACS and not exposed to ACS. In relation to newborns (Table-1) there were no difference between mean gestational age and mean birth weight among two groups. In this study, mean birth weight in ACS exposed and ACS unexposed group were 1665.57±398.53g and 1510.13±377.92g. Mean gestational age were 32.59±1.58and 31.95±1.59 in ACS exposed and ACS unexposed group respectively. Among the enrolled neonates, 55 (67.1%) cases delivered in this hospital were taken as inborn and 27 (32.9%) cases were out born. Among them, 77.3% inborn preterm babies received antenatal corticosteroids where as 22.7% out born babies received ACS. This study result reflected that out born cases suffered more from complications than inborn cases. Because most of the mothers of inborn cases routinely received antenatal corticosteroids during preterm labour, whereas only one-third of outborn cases received the same. With regard to the maternal characteristics (Table-2), it was noted that, in the group exposed to the antenatal therapy, there was a greater number of pregnant women with frequency of pregnancy-induced hypertension and urinary tract infection and premature rupture of membrane. These results reflect the fact that antenatal corticosteroids are more frequently administered in our context to women who presented with problems during the pregnancy that often result to anticipated delivery.This study illustrates that preterm babies who are exposed to ACS had significantly lower incidence of respiratory morbidities than those who are unexposed to ACS. Among ACS exposed group only 9(20.5%) developed respiratory morbidities in comparison to ACS unexposed group 28(73.7).Overall respiratory distress syndrome and pneumonia also were significantly low in ACS exposed group. Percentage of other cause of respiratory distress like transient tachypnea of newborn and delayed adaptation in both group were presented in less frequencies in this study. (Table 3) Respiratory supports in any forms were also needed less in ACS exposed group. Duration of respiratory support more than 48 hours was needed more in ACS unexposed group. (Table-4) Antenatal steroids stimulate the development of type 1 and type 2 pneumocytes which leads to structural and biochemical changes improving both lung mechanics and gas exchange. Type 2 pneumocytes increases surfactant production by producing surfactant proteins and enzymes required for phospholipid synthesis. Other effects of antenatal corticosteroids are induction of pulmonary beta-receptors, which cause surfactant release and absorption of alveolar fluid and induction of fetal lung antioxidant enzymes, and upregulation of gene expression for the epithelial Na+ channel, which is important for absorption of lung fluid after birth.13 Thus ACS acts to reduce respiratory morbidities when administered before 34 weeks. In March 2017, the updated Cochrane systematic review on ACS for accelerating fetal lung maturation for women at risk of preterm birth was published, including 30 trials of 7774 women and 8158 infants. The findings were similar to earlier iterations, showing striking reductions in neonatal respiratory morbidities. 14 It was observed by Meneguel et al. that in their study antenatal corticosteroids had a significant protective role, reducing the occurrence of respiratory distress syndrome in the population studied by more than 50% being similar to what was found in the present study. 15 A retrospective analysis of clinical practice in Poland based on a large sample size of 987 neonates at the gestational age of ≤ 32 weeks from 54 Polish neonatal centers, demonstrated high efficacy of antenatal corticosteroid use in decreasing severe forms of RDS, lower oxygen demand and less invasive ventilation time.16 A total 8 neonates died during hospital course. Most of the causes were due to sepsis and sepsis-related complications. 40 neonates in ACS exposed group and 29 neonates in ACS unexposed group were discharged from NICU. Two infants died before 1st follow-up. BSID III was done at 3rd and 6th months of ageto assess neurodevelopmental status by appropriately trained clinical psychologists. Mean composite score were significantly low in ACS unexposed group. Though in ACS exposed group, the level did not attained normal (Table- 5). At 6 months of age total 47 infants were followed up. At 2nd follow up language and motor composite domains were improved in ACS exposed group than ACS unexposed group though scores were improved in ACS unexposed group as well. Cognition in both group were low which was statistically significant (Table-6) Univariate analysis of factor influencing neurodevelopmental outcome shows only ACS exposure acts as a significantly protective factor. (Table-7) This study demonstrated that ACS exposure can improve neurodevelopmental outcome at 6 months of age though cognition was low in both group. The mechanisms of the beneficial effect of ACS on neurodevelopmental outcomes are not clearly known. Fluorinated glucocorticoids have a broad spectrum of pharmacological properties, which could be beneficial or deleterious. They have anti-inflammatory and antioxidant properties (decreasing the penetration of macrophages through the blood–brain barrier, inhibiting the proliferation and activation of microglia, reducing the release of pro-inflammatory cytokines and promoting maturation of antioxidant systems) which could be beneficial. These properties could explain in particular the significant decrease of white matter injury in the subgroup of 28- to 32-week GA subgroup.17,18 A recent meta-analysis suggested that a single course of ACS in women at high risk for preterm birth appears to improve most neurodevelopmental outcomes in offspring born before 34 weeks of gestation. A single course of ACS was associated with reduced risk for CP (relative risk, 0.68; 95% CI, 0.56–0.82), psychomotor development index score less than 70 (relative risk, 0.83; 95% CI, 0.74–0.93), and severe disability (relative risk, 0.79; 95% CI, 0.73–0.85).19 In a large multicenter prospective study by Sanjay Chawla, et al.20 noted that the protective effect associated with ACS on early childhood neurodevelopmental outcome was dose-dependent. Improved survival without NDI was associated with reduced intracranial hemorrhage or periventricular leukomalacia in the ACS groups. However, the previous Cochrane meta-analysis, 14 had shown no benefit of ACS on childhood outcomes. Lex W Doyle et al. 21 in a follow-up study at 14 years of age born with birth weight less than 1501 grams found better cognitive function assessed by WISC III in children exposed to antenatal steroid, though after adjusting confounder no statistical conclusion was drawn concerning antenatal corticosteroid therapy. This study results shows better effect of ACS on neurodevelopment.
Conclusion
Antenatal corticosteroid therapy effectively reduces respiratory morbidities in preterm neonates born ≤ 34 weeks of gestation. ACS exposure was also associated with improved neurodevelopmental outcomes in all domains compared to the ACS unexposed group, though cognition was low in both groups.
Limitations of the study
-
Small sample size
-
BSID III was not done by single clinical psychologist,
-
Short follow-up period to assess neurodevelopmental outcome.
Recommendation
Conflict Of Interest: Nil
Source Of Funding: Nil
Author Contributions:
Conceptualization: Mosammad Alpana Jahan, Rabeya Sultana, Tarun Kumar Roy, Sanjoy Kumer Dey, M. A. Mannan, Mohammad Kamrul Hassan Shabuj, Ismat Jahan, Sadeka Choudhury Moni , Mohammod Shahidullah.
Writing-original draft: Mosammad Alpana Jahan
Writing-review & editing: Mosammad Alpana Jahan, Tarun Kumar Roy, M. A. Mannan.
All authors contributed to the final version of the manuscript.
All authors read and approved the final manuscript.
Acknowledgment:Care providers of Department of Neonatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Ethical Clearance: This work was supported by the department of neonatology, BSMMU, Dhaka, Bangladesh. Approved by the institutional ethics committee no. 2018/3587; dated March 28, 2018
References:
1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R. National, regional, and worldwide estimates of pre-term birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The lancet. 2012 Jun 9; 379(9832):2162-72.
2. Definitions W. South East Asia Regional Neonatal-Perinatal Database. World Health Organization (South-East Asia Region) Available from-http://www. Newbornwhocc. Org/pdf/database. Pdf. Accessed-February. 2016 Feb;25.
3. NIPORT I. Bangladesh demographic and health survey 2017- 18: key indicators. Dhaka, Bangladesh, and Rockville, Maryland, USA. 2019.
4. Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004 Aug 1; 114(2):372-6.
5. Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? The lancet. 2005 Mar 5; 365(9462):891-900.
6. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet. 2008 Jan 19; 371(9608):261-9.
7. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee opinion no. 677: antenatal corticosteroid therapy for fetal maturation. Obstetrics and gynecology. 2016 Oct; 128(4):e187-94.
8. Gomella TL, Cunningham MD. Neonatology 7th Edition. McGraw-Hill Prof Med/Tech; 2013 Jul 21.
9. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of respiratory distress syndrome in premature infants. Pediatrics. 1972 Oct 1; 50(4):515-25.
10. Foix-L’Hélias L, Marret S, Ancel PY, Marchand L, Arnaud C, FressonJ. Impact of the use of antenatal corticosteroids on mortality, cerebral lesions and 5?year neurodevelopmental outcome
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License