IJCRR - 7(18), September, 2015
Pages: 41-45
Date of Publication: 20-Sep-2015
Print Article
Download XML Download PDF
PHYSIOCHEMICAL PROPERTIES OF SOME PAEDIATRIC FORMULATIONS OF ARTEMETHER -LUMEFANTRINE PRESCRIBED FOR UNCOMPLICATED PLASMODIUM FALCIPARUM MALARIA.
Author: Awofisayo Sunday O., Okhamafe Augustine O., Arhewoh Mathew I.
Category: Healthcare
Abstract:Physicochemical properties bothering on the quality of powder for paediatric suspension (PPS) of artemether-lumefantrine (AL) were evaluated. The moisture content, viscosity, total solid and chemical contents were determined. The assay was analyzed simultaneously for artemether and lumefantrine using high pressure liquid chromatography (chromosil C18 column/ UV detection at 216 nm). Acetonitrile: 25 mM potassium dihydrogen phosphate (70: 30%, v/v) and nevirapine served as mobile phase and internal standard, respectively. Statistical analysis was done using students t-test to compare the parameters for the products at CI, 95%. The artemether and lumefantrine contents varied from 40.3-112.54% and 71.9 \? 91.4%, respectively. The range of values (mean) of moisture content, viscosity, pH and total solid were 2.9-6.9 (4.68)% , 99.1-193.8(124.7) mPa.s, 3.5-7.8 (4.7) and 93.1- 97.1 (95.3) %, respectively. The results showed statistical different outcomes (P < 0.05). PPS products sampled vary widely in their physicochemical properties.
Keywords: Artemether-lumefantrine, Paediatric formulations, Physicochemical properties, Uncomplicated malaria
Full Text:
INTRODUCTION
The incidence of malaria worldwide is estimated to be within the range of 300 – 500 million clinical cases each year with about 90% of these occurring in Africa (WHO, 2013). Malaria is estimated to kill between 1.1 and 2.7 million people worldwide each year, about one million of whom are African children under the age of five (Cesar, 2009). As Plasmodium falciparum causes millions of clinical episodes and infant deaths yearly in Africa, it is therefore of vital importance that antimalarial drugs used for treatments are genuine and of high quality (Amin and Kokwaro, 2007; Awofisayo et al., 2010). The high prevalence of substandard antimalarials in the African retail sector is of great importance in view of their frequent use for fever/malaria treatment (Bapner et al., 1996; WHO, 1999; 2006; Helin-Tanninen, 2001). The past decade has seen increased interest in specific population-targeted and individualized-drug development. Several legislative initiatives in the US (e.g., The Best Pharmaceuticals for Children Act) and Europe (e.g., Paediatric Investigation Plans as indicated in Paediatric Regulation EC 1901/2006), supported by the International Conference of Harmonization (ICH) and World Health Organization, were recently taken to stimulate and improve pharmaceutical care for infants, children and adolescents (EMEA, 2006; Zajieek, 2009; Vandercruyssen et al., 2004). There is need for availability of paediatric formulations of artemether-lumefantrine (AL) that permit accurate dosing and enhance patients’ compliance (USP, 2014). In spite of increased artemether use in treating malaria in endemic areas, the report of therapeutic failure is rising and scientific literature is still limited regarding analytical methods aimed at quantitation of the drug in pharmaceutical products. The United States Pharmacopeia, 2014 (Pingala and Mangaokar, 2013; USP, 2014) contains monographs of pure artemether and the parenteral form. Lumefantrine, the co-formulated. drug in fixed dose artemisinin – based combination therapy (ACT) has been analyzed by a chromatographic method involving the principle of tandem instrumental application with liquid chromatography – mass spectrophotometry (LC-MS) (Khalil et al., 2011). Quality indices of powder for paediatric suspension (PPS) formulations such as viscosity, pH and moisture content that may influence the physical and chemical stability of drug products, such as artemether and lumefantrine, will require systematic evaluation. The multisource nature of drug production may, however, be responsible for any observable physicochemical differences. The aim of this present work was to evaluate the physicochemical factors influencing the quality of PPS of fixed dose AL products sold in Nigeria.
MATERIALS AND METHODS
Chemicals
AL PPS generic products coded (ALA - ALF), sourced from registered pharmacy outlets in Uyo, southeastern Nigeria; nevirapine was used as internal standard (IS), while acetonitrile and potassium dihydrogen phosphate, as mobile phase, were all products of Sigma Aldrich, Germany. Artemether and lumefantrine reference powders were obtained from Qimdis, France. All the reagents employed were of analytical grade.
Physical Observation
The samples were visually examined to assess characteristics such as odour, colour and texture of powder.
Viscosity Determination
To reconstitute the powder products of AL, 20mL of distilled water was added, shaken well and made up to 60 ml mark with water. The viscosity of the reconstituted PPS products was evaluated using a viscometer (Mettler Toledo, Germany). Twenty milliliters of the suspension was placed between the cone and the basal plate at standard temperature condition, 32o C and rotation at 5 rpm for 5 min. The water content of the PPS products was determined using 1 g of powder for the analysis in a moisture analyzer and heating up to 105o C. Measurement was performed in triplicates for each drug product.
pH Determination
The pH of the reconstituted products was measured (n = 3) by dipping the probe of the device directly into the reconstituted products using a pH/mV meter (Mettler Toledo, Germany) at temperature of 25o C.
Total Solid
The reconstituted products were shaken up. After flocculation, 20 mL samples were taken with a pipette from the same depth and added to a porcelain dish of known weight, W1. This was evaporated to dryness by placing the dish with its content first on a water bath and subsequently in an oven (Galenkemp No. 335, England). The samples were intermittently cooled in a dessicator and weighed until a constant weight, W2, was obtained. The difference in weights (W2- W1) was calculated and the total solid percent determined from equation 1.
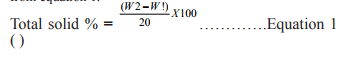
Chemical content determination
The internal standard was prepared by dissolving 20 mg of nevirapine in 10 mL of deionized water and made up to mark in a 1 L volumetric flask resulting in 20 mg/ml solution. Approximately 250 mg and 1500 mg of artemether and lumefantrine reference powders, respectively, were transferred into a 100-mL volumetric flask and made up to mark. The solutions were sonicated and then diluted to 1 L in a volumetric flask to produce 0.25 and 1.5 mg/mL stock solutions, respectively. The stock solutions produced were further diluted with acetonitrile: acetic acid (99: 2, v/v) to obtain the working solutions. The working solutions were spiked with IS solution to give uniform amount of the IS in the working solution. One sample was taken from one bottle and three bottles were sampled. High pressure liquid chromatographic (HPLC) system was used to assay the samples (HPLC Peak 7000 system with analytical chromosil column C18, 250 x 46 mm, Rheodyne manual samples injectors, Germany).
Data Analysis
All statistical analyses were performed using SPSS version 13.0. The values for each parameter evaluated were compared among brands and with the reference product coded ALA using one sample t-test and statistical difference was taken at P<0.05.
RESULTS AND DISCUSSION
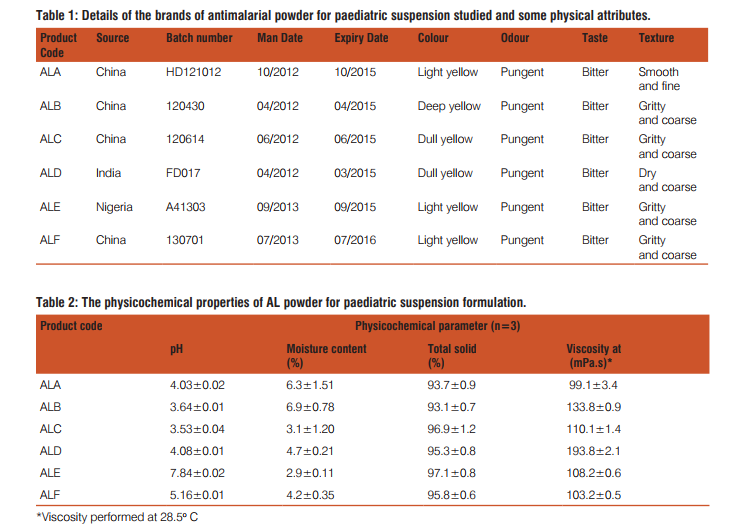
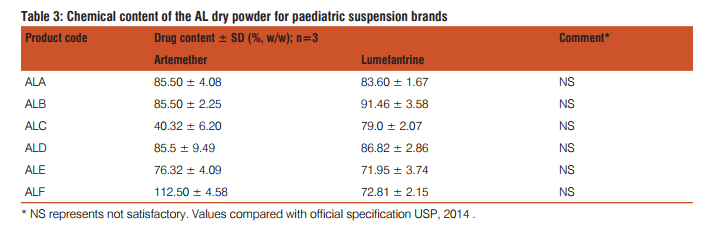
There were a total of 12 registered/approved PPS formulations in Nigeria at the time of this study. A sample size of 6 PPS brands were randomly selected from the registered products and the details of the products are listed in Table 1. Visual observations of the physical characteristics (i.e., colour, texture, taste and odour) of the products are also reported in Table 1. The physicochemical parameters that were used to assess the quality of the PPS products are viscos-ity, moisture content, total solid and pH are laid out in Table 2. The mean values for the replicate sampling together with the standard deviation are presented. The chemical content data for the various brands are reported in Table 3 and they showed good linearity with regression coefficient of 0.991 and 0.999 for artemether and lumefantrine, respectively. AL has become the most widely used antimalarial drug treatment in the world following WHO recommendation (Sridhar et al., 2010). This study, therefore, examined the quality of the circulating PPS formulation in the light of the wide prescribing in the study area. The trade mark owners of AL market the 20/120 mg dispersible tablets for paediatric use, and hence product ALA was taken as the reference, being the most widely prescribed brand. The widespread news of marketing of substandard drug products in the African continent where incidentally malaria bears a high toll will require close monitoring of antimalarials. Wide differences were observed in the physical characteristics of the PPS products. The colour, odour and texture of the products were strikingly different, showing varying shades of yellow colour and coarseness in the powder feel. Visual grittiness of the powder in the products gives an indication of possible high levels of moisture content. This is expected as there is no documented standard operating procedure (SOP) for the sale AL drug products. The sourcing of antimalarials drugs marketed in Nigeria, including the evaluated products majorly stems from Asia in particular, India and China as shown in Table 1. Variations in the physical features in the sampled products may also be attributed to the varying levels of drug degradation in the products. Table 2 expresses the varying levels of moisture content in the products. Nearly all drugs contain at least some residual moisture,, however, excessive or deficient moisture content of a substance can adversely impact on the physicochemical properties of a drug product (Lavoie et al., 2002). The propensity of microbial growth in drug products depends on their water content. The presence of moisture in any processing environment is unavoidable, and it is absolutely necessary, therefore, to control moisture parameters during the production of PPS products. The range of moisture content in the sampled products is higher than the stipulated range in most drug monographs. For example, the US Pharmacopoeia stipulates a value of not more than 1% in most cases (USP, 2014). The other parameters such as pH of the PPS products also varied widely among the brands. The viscosity of the reconstituted products similarly varied widely indicating the likelihood of differences in chemical reactivity of the constituent drug components in the products (Lavoie et al., 2002). The chemical content of the active ingredients in the PPS formulations were simultaneously determined by HPLC. Earlier studies on simultaneous analysis of artemether and lumefantrine have been performed using LC-MS (Khalil et al., 2011). The method employed here was a validated procedure modified from the USP procedure. The simultaneous determination of artemether and lumefantrine components using IS is an advantage as the components are determined and variations in determination are ruled out. The chemical content of the various brands indicate levels lower than the labeled content. Substandard antimalarial products in Africa have been frequently reported. The findings of the present study is yet another indication that poor quality antimalarial products still abound in the Nigerian market and that the poor physicochemical properties of products might have adversely affected physicochemical instability resulting in the lower contents of the active ingredients (Lavoie et al.,2002).
CONCLUSION
Many antimalarial products in use have been reported to fall below the regulatory standards due to deliberate counterfeiting and/or inadvertent poor quality presentation due to lack of standard operating procedure (SOP) for the manufacture of the drugs. The PPS formulations of AL studied have also confirmed that gross inconsistencies exist in the physicochemical properties of the several marketed products. Findings from this study therefore serve as an indicator to one of the causes of the issue of sporadic development of drug resistance.
AUTHORS’ STATEMENT
The authors have no conflicts of interest to disclose.
References:
1. Amin A.A., Kokwaro G.O. 2007. Antimalarial drug quality in Africa. J Clin PharmTherap, 32: 429–440.
2. Awofisayo S.O., Willie E., Umoh E. 2010. Quality control evaluation of multi-source artemether- lumefantrine tablets prescribed for uncomplicated multi-drug resistant malaria. Ind J Nov Drug Del, 4: 153-157.
3. Bapner J.S., Tripathi C.D., Tekur U.1996. Drug utilization pattern in third world. Pharmaco-economics, 9:286-294.
4. Cesar I.C, Pianetti G.A. 2009. Quantitation of artemether in pharmaceutical raw material and injections by high performance liquid chromatography. Braz J Pharm Sci., 45: 738-742.
5. European Medicine Evaluation Agency (EMEA). 2006. EMEA/ CHMP/PEG/19481/2005. Reflection paper formulation of choice for the paediatric population; EMEA, London.
6. Food and Drug Administration (FDA). 2007. Food and Drug Administration Amendments Act, FDA, Silver Spring.
7. Helin-Tanninen M., Naaranlahti T., Kontra K., Wallenius K. 2007. Enteral suspension of nifedipine for neonates. Part 1. Formulation of nifedipine suspension for hospital use. J Clin Pharm Therap., 26: 49–57.
8. Khalil I.F., Abildrup U., Alifrangis L.H. 2011. Measurement of lumefantrine and its metabolite in plasma by high performance liquid chromatography with ultraviolet detection. J Pharm Biomed Anal., 54:168–172.
9. Lavoie F., Cartilier L., Thibert R. 2002. New methods characterizing avalanche behaviour to determine powder flow. Pharm Res.,19: 887-893.
10. Pingala S.G., Mangaonkar K.V. 2013. Quantification of lumefantrine in human plasma using LC-MS/MS and its application to bioequivalence study. J Pharm., 13:1-8.
11. Sridhar B., Rao K.H., Srinivas T.V. 2010. A validated reverse phase HPLC method for the simultaneous estimation of artemether and lumefantrine in pharmaceutical dosage forms. Int J Adv Pharm Sci., 1: 95-99.
12. United States Pharmacopoeia. 2014. The National Formulary, USP 38 NF13; United States Pharmacopeia Convention, Inc.: Rockville, MD, USA.
13. Vandercruyssen K., D’Hont M., Vergote V. 2004. LC-UV/MS Quality analytics of paediatrics artemether formulations. J Pharm Anal., 4 (I): 37 – 52.
14. World Health Organization. 2006. Counterfeit Medicines. Facts sheet no 275. 2006. Revised February 2006.
15. World Health Organization. 1999. Counterfeit Drugs; Guidelines for the development of measures to combat counterfeit drugs. Geneva: WHO, pp 1 – 60.
16. World Health Organization. 2013. World Malaria Report; Geneva, Switzerland.
17. Zajieek A. 2009. The national institutes of health and the best pharmaceuticals for children act. Paed Drugs., (II): 45 – 47.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License