IJCRR - 7(18), September, 2015
Pages: 19-24
Date of Publication: 20-Sep-2015
Print Article
Download XML Download PDF
EFFECT OF SETTING ON THE PROPERTIES OF PROTEINS FROM THREADFIN BREAM (NEMIPTERUS JAPONICUS) FISH MINCE
Author: B. U. Supreetha, M. V. Chandra, B. A. Shamasundar
Category: Healthcare
Abstract:In the present investigation effect of setting on the properties of proteins and gel forming ability from threadfin bream (Nemipterus japonicus) fish mince has been assessed. The term setting refers to obtaining a soft elastic gel upon incubating the fish mince mixed with sodium chloride (NaCl) at less than 40 \?C for a known duration. When the set meat is subjected to heat processing at 90 \?C for 45 min, yields a gel with higher strength. The setting was carried out at a temperature at 35 \?C for different durations. The gel strength of the gel obtained from set meat at 35 \?C for 45 min was found to be 643.8 while obtained from unset meat was 264.5 g.cm. The setting of fish mince was accompanied by decrease in protein solubility, free-sulfhydryl content, Ca2+-ATPase
enzyme activity and viscosity.
Keywords: Threadfin bream mince, Setting and gel forming ability
Full Text:
INTRODUCTION
Gelation is a process and gel is an end product. In order to improve the gel forming ability of proteins from fish species a process known as setting is employed in many of the fish processing and surimi industry. The term surimi refers to separated fish mince subjected for water washing, partially dehydrated and mixed with cryoprotectants, frozen and frozen stored (Yin et al., 2014). Surimi is used to prepare different analogue products, like crab leg sticks, lobster/shrimp moulded products, kamaboko and related products. The characteristic features of these products are its texture which is viscoelastic in nature and are contributed by myosin, the major myofibrillar protein fractions (Xiang et al., 2011). The setting is a process wherein, the fish mince is mixed with sodium chloride and incubated at a temperature below 40 ?C for certain duration resulting in the formation of a translucent, soft elastic gel (Benjakul et al., 2004; Liu et al., 2007). This process is called ‘setting’ and the meat is referred to as set meat. Cross-linking of myosin heavy chain is attributed to transglutaminase enzyme. The enzyme catalyzes the cross-linking reaction with the formation of isopeptide, ε-γ-glutamyl-lysine (EGL) linkages which form soft elastic gel (Binsi and Shamasundar, 2012). When the set meat is subjected to heating at 90 ?C for 45 min duration, yields a strong thermally induced visco elastic gel (Roussel and Cheflel, 1990) as compared to unset meat. Such inter and intra molecular covalent cross-linking of myosin molecules result in higher gel elasticity. The setting ability is species specific and the presence of endogenous TGase enzyme will determine the extent of process (Benjakul et al., 2004). Apart from the cross-linking of myosin heavy chain (MHC) during setting process may initiate certain changes in the properties of proteins like solubility, free-sulfhydyl content and viscosity (Niwa et al., 1991). As water washing is an essential step in the surimi preparation where many soluble protein fractions including TGase are removed. Such water washed mince (surimi) may have a little setting ability. It is common to add TGase enzyme from different source to initiate the setting process when surimi is used as base material for the preparation of products (Nowsad et al., 1997). It will be of interest to study the gel forming ability of fish mince in relation to setting process without washing the mince. It has been reported that unwashed mince will have TGase enzyme and can be made use for setting and enhancing the gel forming ability (Lanier, 2000). In the present investigation the effect of setting on properties of proteins from threadfin bream (Nemipterus japonicus) fish mince have been studied.
MATERIALS AND METHODS
Materials
Fresh threadfin breams (Nemipterus japonicus) were procured from the local fish market, Mangalore, India. The fishes were washed with chilled potable water (3-4 ?C) and iced in the ratio of 1:1 (fish: ice) and transported to laboratory. The fishes were dressed to remove head and entrails and washed with chilled water. The semi dressed fishes were used for further analyses.
Methodology
Setting experiment
Meat was separated from the threadfin bream fishes manually avoiding scales and bones and used for the study. The separated meat was macerated in a pre-cooled pestle and mortar where temperature was maintained at 4 ?C using ice bath. Sodium chloride at 2.5 % (w/w) was mixed with fish mince and macerated for 5 min to get a homogenous sol. The sol was subjected for setting at 35 ?C for 15, 30 and 45 min setting duration in an incubator which was thermostatically controlled (Orbitek, Scigenics biotech Company, Chennai, India).
Gel preparation
About 100 g of set meat (as mentioned above) was stuffed into krehalon casing (copolymer of vinyledene chloride and vinyl chloride) of 50 mm x 250 mm (dia x length) using a hand stuffer. The stuffed casings were sealed with aluminium clips using a ringer machine (Seinco, TX/ 8037, Barcelona, Spain). The heat processing was carried out at a temperature of 90 ± 2 ?C for 45 min to get thermally induced gels. The gels were cooled at room temperature and stored in a refrigerated condition at a temperature of 6 ± 2 ?C overnight prior to measuring the gel strength. The gels from meat without setting were prepared and served as control.
ANALYSIS
Protein solubility
The solubility of total proteins in the unset (control) and set threadfin bream fish mince was assessed using high ionic strength buffer (phosphate buffer 50mM, pH 7.5 containing 1.0 M sodium chloride) as solvent. This solvent here afterwards will be referred to as extraction buffer (EB).The ratio of fish mince to extraction buffer used was 1: 10 (mince: buffer) and homogenized using laboratory homogenizer (Ultra-Turrax T125, Janke and Kunkel, Staufen, Germany) at 9000 rpm for 2 min. The slurry was centrifuged at 12000 x g at 4 ?C for 30 min using high speed refrigerated centrifuge (Sorvall Legend XTR centrifuge, Thermo Fisher Scientific, New Hampshere, USA). The protein concentration was determined according to the method as described by Lowry et al. (1951). The solubility of the protein was expressed as mg/ ml.
Free sulfhydryl content Free sulfhydryl content was determined according to the method as described by Ellman (1959). The absorbance was measured at 412 nm was using double beam spectrophotometer (Spectro UV- VIS, Labomed, Inc., Los Angeles, CA, USA). The free sulfhdryl content was expressed as mM/g of meat.
Calcium activated ATPase activity Calcium activated ATPase activity of mince was determined according to the method as described by Noguchi and Matsumoto (1970) and expressed as µmol of Pi/g meat/min at 27 ?C. The inorganic phosphorus released was estimated according to the process as described by Tausky and Shorr (1952).
Sodium do-doecyl sulphate Poly acryl amide gel Electrophoresis (SDS - PAGE) Dialysis: In order to remove the sodium chloride in the set meat, dialysis was carried out. About 5.0 g of set mince meat was placed in a dialysis tubing (Sigma, Seamless dialysis tubing), having a width of 23 mm and dia of 15 mm. Dialysis was carried out at 4 ± 2 ?C using deionised water. The duration of dialysis was 6-8 h with frequent change of water for complete removal of sodium chloride. The meat free from sodium chloride was used for SDS-PAGE analysis. The method followed was as described by Laemmlli et al. (1970). The molecular weight of the protein bands obtained in the sample was approximated by measuring the relative mobility of the standard protein molecular weight markers (high molecular weight markers from Sigma, St. Louis, MO, USA).
Viscosity Viscosity was determined using rotational viscometer (Brookfield Viscometer DV - II + Pro, Brookfield engineering labs, inc., Middleboro, U.S.A) at a constant temperature of 25 ± 1 ?C. The total proteins were extracted using extraction buffer as described previously. The supernatant obtained was diluted using extraction buffer to get a protein concentration of 2mg/ml. A known quantity of protein solution (7ml) was used for viscosity measurement. The sample was taken in sample holder and spherical spindle (SC - 18) was immersed in sample holder and attached to viscometer. The sample was equilibrated to the desired temperature (25 ±1 ?C) using circulatory constant temperature water bath (CB 2000 V, Cyber lab Corporation, Mumbai, India). The rotation of the spindle was carried out at 100 rpm and corresponding viscosity was recorded and expressed in mPa.s.
Gel strength measurements Gel strength of the gel obtained from unset (control) and set mince was measured by using Texture profile analyser (TA - XT plus Stable Micro System, Surrey, England) with 50 kg of load cell. Prior to analysis, the refrigerated stored samples were equilibrated to ambient temperature for 30 min. A spherical probe with 5 mm dia was used for penetration at a constant test speed of 1.1 mm / sec with 10 g of trigger force and target distance of 15 mm. The parameters measured were breaking force (g) and breaking distance [deformation (cm)]. The gel strength was calculated by multiplying breaking force x deformation and expressed as g.cm.
Statistical analysis To know the effect of setting on the physicochemical properties of proteins from threadfin bream (Nemipterus japonicus) fish mince and the gel strength, the statistical analysis was carried out. Analysis of variance (ANOVA) was tested using SPSS (version 21) software package (IBM SPSS). The test of significance was analysed using Tukey’s test at a level of 0.05.
RESULTS AND DISCUSSION
Protein solubility: The protein solubility of unset and set meat in EB as a function of setting duration at 35 ?C is given in Fig. 1A. The protein solubility decreased with increase in setting duration. The setting process initiates the formation of ε-(γ-glutamyl) lysine (EGL) isopeptide, which is a covalent bond and perhaps would have contributed for reduction in solubility. Decrease in solubility of proteins as a function of setting duration has been reported for bigeye snapper, threadfin bream, baracuda and bigeye croaker (Benjakul et al., 2003).
Free sulfhydryl content: The free-sulfhydryl content of fish mince as a function of setting is given in Fig. 1B. A concomitant decrease in free-SH content is an indication of the formation of disulfide (-S-S-) bonds. The intermolecular disulfide bonding during setting is a result of oxidation of sulfhydryl groups in the presence of oxidants or metal ions (Ellman, 1959). From the results it is evident that the reduction in the solubility of protein during setting has been contributed by cross-linking of MHC through isopeptide and disulfide bonds.
Calcium activated ATPase activity: Any conformational changes in proteins initiated by aggregation will be reflected in the Ca2+-ATPase enzyme activity of myosin molecule. The Ca2+- ATPase enzyme activity of the fish mince as a function of setting duration is given Fig. 1C. The reduction in Ca2+- ATPase enzyme activity was found to be significant (P < 0.05) with setting duration and it could be attributed to changes in the conformational status of myosin molecules as affected by setting process. The cross-linking of MHC is primarily an aggregation process involving globular head (Samejima et al., 1981). The ATPase enzyme activity is related to 95 KDa of globular head and it is reasonable to expect that cross-linking has altered the conformation of this particular site resulting in reduced Ca2+-ATPase enzyme activity.
Viscosity: The viscosity of total protein from unset (control) and set at different durations were done at the protein concentration of 2 mg/ml. The results are given Fig. 1D. The viscosity of unset threadfin bream was 2.98 mPa.s. Nearly 2.6 fold decrease in viscosity values was recorded at the end of 45 min of setting. Viscosity is an intrinsic property of protein molecules chiefly dependent on the length and diameter ratio of the molecule. The probable reason for decrease in viscosity as a function of setting could be due to the nature of protein could get into solvent during extraction. It is well known that aggregated protein especially with formation of iso-peptide and disulfide bonds it may not be possible to extract in EB. The initial high value of viscosity in the unset meat includes all the fractions of the protein whereas in the set meat it may be protein fractions not involve in setting. Hence, the decrease in viscosity values is due to dissociation of protein which is not involved in setting.
Sodium do-doecyl sulphate Poly acryl amide gel Electrophoresis (SDS - PAGE): The SDS-PAGE pattern of total proteins from unset (control) and set mince at 35 ?C for 30 and 45 min setting duration is given in Fig. 2. The SDS-Page pattern of unset meat indicated multiple bands in the molecular weight range of 205-25 KDa (lane B). The 205 KDa indicates myosin heavy chain (MHC). The pattern of set meat clearly indicated a decrease intensity of MHC and a band on the top of the gel which represents cross-linked MHC (lane C and D). The decrease in intensity of MHC band is an indication of aggregation and hence will not be observed in the pattern. The low-molecular weight fractions in the range of 66- 24 KDa appear to have not been affected by setting process.
Gel strength measurements: The gel strength of thermally induced gel from unset (control) and set mince is given in Table 1. The gel from unset (control) mince recorded a value of 264 g.cm, while the gel from mince set at 35 ?C different durations the values were in the range of 492-643 g.cm. The results suggest that the gel strength values of gel were proportional to setting duration. This can be attributed to polymerisation of MHC by the action of endogenous TGase.
CONCLUSION
The gel forming ability of unwashed threadfin bream (Nemipterus japonicus) fish mince could be enhanced by setting process at 35 ? C for 45 min. The setting process initiated aggregation reaction as indicated by reduction in free-sulfhydryl content and solubility. The aggregation / cross-linking of MHC are further confirmed by SDS-page pattern.
ACKNOWLEDGEMENT
The financial assistance from European Union, Brussels [Grant No: 289282-Secure fish] for carrying out this work is gratefully acknowledged.
Data are mean of triplicates and value in parenthesis are standard deviation, n=3. Different letters denotes the significant differences among treatments (P < 0.05).

Figure 1 A: Protein solubility of threadfin bream (Nemipterus japonicus) mince set at 35 °C for different durations in high ionic buffer
B: Free- sulfhydryl content of threadfin bream (Nemipterus japonicus) mince set at 35 °C for different durations
C: Ca2+-ATPase enzyme activity of threadfin bream (Nemipterus japonicus) mince set at 35 °C for different durations
D: Viscosity of threadfin bream (Nemipterus japonicus) mince set at 35 °C for different durations at 2mg/ml protein concentration\
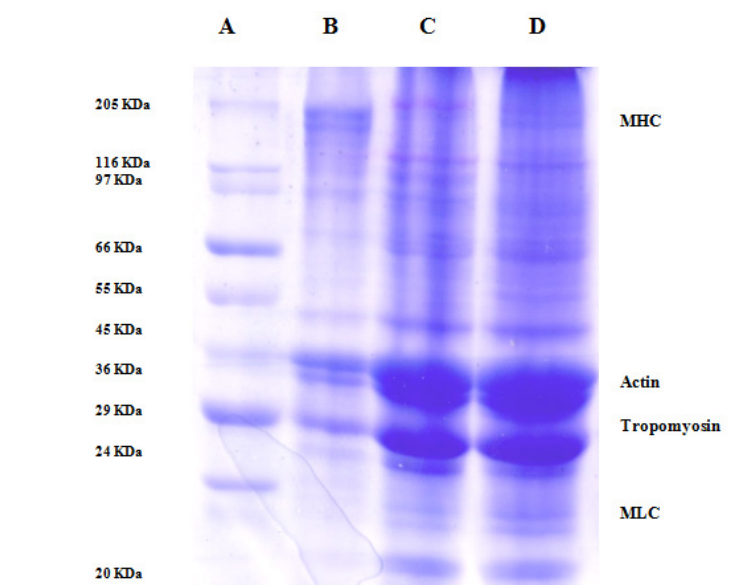

Figure 2: SDS-PAGE pattern of threadfin bream (Nemipterus japonicus) mince set at 35 °C temperature for 30 and 45 min setting durations Lane
A: Molecular weight markers Lane
B: Threadfin bream mince unset (control) Lane
C: Threadfin bream set at 35 °C 30 min Lane
D: Threadfin bream set at 35 °C 45 min
References:
1. Benjakul S., Visessanguan W. and Pecharat, S. 2004. Suwari gel properties as affected by transglutaminase activator and inhibitors. Food Sci. Nutr., 22:27-107.
2. Binsi P. K. and Shamasundar B. A. 2012. Purification and characterisation of transglutaminase from four fish species: Effect of added transglutaminase on the viscoelastic behaviour of fish mince. Food Chem., 132 (4):1922-1929.
3. Ellman, G.L. 1959. Tissues sulfhydryl groups. Arch. Biochem. Biophys., 82: 70-77.
4. Laemmlli U.K., 1970. Cleavage of structural protein during assembly of the head bacteriophage T4. Nature., 227: 680-685.
5. Lanier T.C. 2000. Surimi gelation chemistry. In: J.W. (Ed) surimi and surimi seafood. Marcel Dekker , New York., pp 99-145.
6. Liu R., Zhao S., Xiong S., Xie B. and Liu H. 2007. Studies on fish and pork paste gelation by dynamic rheology and circular dichroism. J. Food Sci., 72: 399-403.
7. Lowry O.H., Rosebrough, N.J., Farr, A.L. and Randall R.J. 1951. Protein measurement with folin phenol reagent. J. Boil. Chem., 193(1): 256-275.
8. Niwa E., Nowsad, A.A and Kanoh, S. Changes in viscoelastic properties of salted fish flesh sol during its low temperature setting. Nippon suisan Gakk., 57 (12): 2333-2336.
9. Noguchi S. and Matsumoto, J. J. 1970. Studies on the control of the denaturation of the fish muscle proteins during the frozen storage. I. Preventive effect of Na–glutamate. Bull. Jap. Soc. Sci. Fish., 36: 1078-1087.
10. Nowsad A.K., K Anoh S and Niwa E.1997. Phscico-chemical protperites of a Suwari gel from Alaska Pollack surimi with iodoacetic acid added. Bull.Fac. Bioresources, Mie Univ.,13: 25- 31.
11. Roussel H. and Cheflel J.C. 1990. Mechanism of gelation of sardine protein influence of thermal processing and various additives on the texture and protein solubility of kamaboko gel. Int. J. Food Sci. Technol., 25: 260-280.
12. Samejima K., Ishioroshi, M., and Yasui T. 1981. Relative roles of the head and tail portions of the molecule in heat-induced gelation of myosin. J. Food Sci., 46: 1412-1418.
13. Tausky H. H. and Shorr E. 1952. A micro colorimetric method for determination of inorganic phosphorous. J. Biol. Chem., 202: 675-685.
14. Xiang, D.S and Holley R.A. 2011. Factors influencing gel formation by myofibrillar proteins in muscle foods. Comprehensive Rev in Food Sci and Food Safety., 10: 33-51.
15. Yin T. and Park J.W . 2014. Effects of nano-scaled fish bone on the gelation properties of Alaska Pollock surimi. Food Chem., 150: 463-468.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License