IJCRR - 14(6), March, 2022
Pages: 09-16
Date of Publication: 15-Mar-2022
Print Article
Download XML Download PDF
\a-Amylase Inhibitory, Immunomodulatory, Phytotoxic, Antifungal Activities and Phytochemical Screening of Medicago Denticulata
Author: Imran Ahmad, Samina Afzal, Muhammad Atif Shahzad
Category: Healthcare
Abstract:Introduction: Plants are an alternative source for the management of a number of human disorders because of diverse metabolites.
Aims: Current study targets to probe underexplored therapeutic potentials of Medicago denticulata (M. denticulata) through phytochemical and biological assays.
Methods: The dried whole plant (aerial and root parts) was extracted in succession with dichloromethane (DCM) and methanol by simple maceration process and designated with codes MDWD and MDWM respectively.
Results: The phytochemical investigation uncovered the occurrence of saponins, terpenoids, tannins, phenols and flavonoids in the extracts. MDWD fraction unveiled the highest total phenolic contents 54.47 \?g GAE / mg dry weight (DW), The total reducing potential 58.125 \?g AAE / mg DW and total antioxidant capacity 81.71 \?g AAE / mg, while MDWM fraction revealed maximum flavonoids content 16.72 \?g QE / mg DW. The phytotoxicity of methanol and DCM extracts exhibited 73% and 63% growth regulation respectively at concentration of 1000 \?g/mL. Extracts revealed antidiabetic activity through \a-amylase inhibition. The maximum inhibition i.e. 116.91% was detected at the concentration of 1000 mg / mL by DCM extract.
Conclusion: Dichloromethane and methanolic extracts of M. denticulata are well-thought-out as the potential source of antioxidants and could provide immunomodulatory, antifungal, phytotoxic and a-amylase inhibition activities.
Keywords: Antioxidant, Flavonoids, Immunomodulatory, Medicago, Phenols, Phytochemical
Full Text:
INTRODUCTION
The assorted plant kingdom of the world is generally acknowledged for its medicinal significance. Potential medicinal constituents of natural plants have significantly contributed in the development of many herbal therapies used for several diseases around the world.1The herbs have been utilized by all cultures for quite a long time. Each area of the world uses herbs indigenous to that area.2 The remedies derived from plants play very important role in the traditional medicine system, like Ayurveda, Egyptian and Chinese, which represents their recurrent usage till today.3 Eastern area of Mediterranean have been recognized throughout generations with a rich record of the natural medicinal herbs.4 A striking number of new drugs had been developed from terrestrial plants being utilized as medicines in China, Egypt, India and Greece from antiquated time. There is a prediction that plants can give potential bioactive compounds for development of new ‘leads’ for example, vinblastine and vincristine used in therapy of cancer.5,6
Regardless of much advancement made in synthetic drug research, natural plants and their products are yet considered as significant sources of the medicaments and have wide use in pharma industry.7, 8 From last few years, there has been increasing curiosity around world to investigate novel and hidden potentials of the traditional herbal preparations.9 Recent research in the drug discovery from medicinal plants includes multidimensional approach through combination of biological, phytochemical, botanical, and other molecular procedures. The medicinal plants drug discovery keeps on giving new significant leads against different pharmacological targets.10
Pakistan has been blessed through botanical wealth with diverse flora. Generally in deserts, western and northern seashore and mountain areas of Pakistan approximately 6000 taxa comprising the flowering plants were accounted.11 To best of our knowledge, the most of traditional plants in the vicinity have not been characterized up till now to demonstrate their biological and phytochemical potentials. Thus, current study has been concentrated predominantly on phytochemical and pharmacological profiling of M. denticulata.
Genus Medicago
The genus Medicago L. belonging to family Fabaceae (subfamily Papilionoideae, tribe Trifolieae) comprises of fifty species, of which Medicago sativa L. (alfalfa) is renowned. These species are perennial or annual herbs and rarely shrubs.12 Medicago polymorpha (Burr medic) is a common, polymorphic annual legume of Mediterranean origin. It has important value in many dry land farming systems as a self-reseeding and very effective nitrogen fixer.13 Fresh leaves of Medicago polymorpha cooked in water are taken orally to treat indigestion and constipation.14
MATERIALS AND METHODS
Plant Collection
Plant was gathered from periphery of Bahauddin Zakariya University Multan, Pakistan, during months of March and April. The plant was identified as Medicago denticulata(synonyms were Medicagopolymorpha and Burr Medic) by Dr. Zafar Ullah Zafar, The institute of pure and applied biology, Bahauddin Zakariya University Multan, Pakistan. The voucher no. R.R.Stewart.F.W.Pak.413 was provided to that plant.
Extraction of plant material
For effective extraction, the whole plant material was dried at a room temperature for a period of 3 to 4 weeks before it was comminuted to coarse powder and weighed. Extraction of that powdered material was done through simple maceration procedure. Weighed quantity of that powdered plant was soaked in a measured volume of the dichloromethane (DCM) solvent for 24 hours in an extraction bottle before filtration was carried out. Above process was repeated 3 times with dichloromethane solvent. Then extraction of this marc was completed in a similar way by using methanol solvent. To attain crude extract, Dichloromethane and methanol extracts were subjected to dryness in a rotary evaporator (Buchi, Switzerland) under the reduced pressure. The dichloromethane (12 g) and methanol (23.6 g) extracts collected in the separate sample bottles were labeled with codes as MDWD and MDWM respectively.
Qualitative Phytochemical Analysis
Preliminary phytochemical examination for extracts was completed as described by.15
Quantitative Phytochemical Analysis
Total phenolic along with flavonoid contents were measured by following described procedures.
Estimation of total phenolics
Total phenolic contents (TPC) of M. denticulata fractions were determined using a method based on the study of 16 with a slight change. Briefly, 20 µL of the each fraction was put in the 96-well plate, followed through adding 90 µL of diluted FolinCiocalteu reagent in the each well plate. After incubating that mixture for five minutes at a room temperature 6% sodium carbonate solution (90 µL) was added and then incubated again for 1 hour at a room temperature. The absorbance was documented on microplate reader at 630 nm wavelength. Value of total phenolic content was determined as gallic acid equivalent (GAE).17
Estimation of total flavonoids
Aluminum chloride based colorimetric technique was utilized for quantification of total flavonoid content (TFC), using quercetin as a standard.18 Concisely, the reaction mixture comprising 20 µL of the sample solution (20 mg/mL DMSO), 10% aluminum chloride (10 µL), 1M potassium acetate (10 µL), and 160 µL of distilled water was incubated for half an hour at room temperature. Then, spectrophotometric absorbance of this mixture was determined at 415 nm wavelength and total phenolic content was communicated µg QE/mg DW.3
Total Antioxidant Capacity (Phosphomolybdate Assay)
For determination of antioxidant capacity of samples Phosphomolybdate technique was utilized.19 The assay mixture (1000 microliter) containing sodium phosphate (0.028 M), H2SO4 (0.6 M) and 0.004 M ammonium molybdate was transferred to the sample tubes holding 100 µL test sample. The mixture samples were cooled after heating for 1.5 hours at 95 ?C hot water bath. The absorbance of reaction mixture was recorded at 765 nm. As reference standard ascorbic acid was utilized.20
Total Reducing Power (Potassium Ferricyanide Colorimetric Assay)
Reducing powers of the extracts were evaluated as per process described previously by.21, 22 Briefly, the mixture containing 200 µL of test extract (4 mg/mL DMSO), 0.2 mol/L phosphate buffer having pH value 6.6 (400 µL and) and 1% potassium ferricyanide [K3Fe(CN)6] were incubated at temperature 50?C for twenty minutes. About 400 µL trichloroacetic acid (10%) was added to above mixture and then centrifuged at 3000 rpm for ten minutes at a room temperature. A 500 µL solution from upper layer, distilled water (500 µL) and 100 µL FeCl3 (0.1%) were mixed. The absorbance was noted down at 700 nm wavelength and an increased value of absorbance of the mixture is related with increased reducing power. For the preparation of blank instead of the extract a 200 µL of DMSO was added to the above-mentioned reaction mixture. Reducing powers of those samples were stated as per µg ascorbic acid equivalent per mg plant dry weight (µg AAE/mg DW).23
BIOLOGICAL EVALUATION
α-amylase inhibitory assay
The assay was performed with the help of a modified procedure described by24 An amount 250 µL of the extract solution was placed in the tube followed by the addition of equal volume of sodium phosphate buffer (0.02M) having pH=6.9 comprising 0.5 mg/mL of ?-amylase solution. After pre-incubation of this solution at 25?C for ten minutes, an amount 250 µL of the starch solution (1%) in a sodium phosphate buffer (0.02M) with pH=6.9 was added in different intervals of time and after that incubation was done for 10 minutes at 25?C. The reaction was ended through the addition of 500 µL of dinitrosalicylic acid (DNS) reagent. Tubes were incubated in the boiling water for five minutes and then cooled at room temperature. After diluting with distilled water (5 mL), the absorbance of the reaction mixture was measured by the use of a spectrophotometer at 540 nm wavelength. By the use of the same procedure, control was set by substituting extract by distilled water. Then ?-amylase inhibitory potential was determined as %inhibition by the following given formula.25

Antifungal assay
Agar tube dilution assay was performed for screening of extracts about their in vitro antifungal action against Aspergillus flavus, Candida albicans, Fusarium solani and Microsporum canis. Test samples were dissolved in dimethyl sulfoxide (DMSO) to prepare stock solution. Sabouraud dextrose agar (4%) was prepared in distilled water. After dissolving the contents with the help of a magnetic stirrer, known quantity was distributed into the screw-capped tubes. These tubes holding media were then autoclaved for 15 minutes at 121N.C. Then after cooling these tubes to 50?C, test samples were taken from the stock solution into a non-solidified Sabouraud agar medium. Then at room temperature, the tubes were permitted to solidify. Inoculation of each tube was done using a piece of the inoculum (4mm diameter) detached from 7 day old fungi growth culture. Incubation of the tubes containing culture was done for growth at an optimal temperature 28–30.C for seven days. Humidity (40-50%) in the incubator was constrained by the placement of an open pan filled with water. These cultures during incubation were observed twice in a week. The standard drugs in this assay were Miconazole and Amphotericin B. % Inhibition of fungal growth was determined by the formula given.26
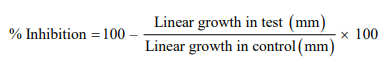
Immunomodulatory assay
In Luminol-enhanced chemiluminescence assay a 25 µL of diluted whole blood HBSS++ (Hanks Balanced Salt Solution, comprising magnesium chloride and calcium chloride) [Sigma, St. Louis, USA] was incubated along 25 µL extract sample that have a concentration of 50µg/mL. The control wells contain cells and HBSS++ only, no extract sample. The performance of this test was made in white half area of the 96 well plates [Costar, NY, USA], which was incubated for fifteen minutes at 37?C in the thermostat chamber of a luminometer [Labsystems, Helsinki, Finland]. After incubation, an addition of a 25 µL of serum opsonized zymosan (SOZ) [Fluka, Buchs, Switzerland] and 25 µL of intracellular reactive oxygen species (ROS) perceiving probe, luminol [Research Organics, Cleveland, OH, USA] was done in each well, with the exception of blank wells that contain HBSS++ only). The level of ROS was documented in the luminometer in terms of a relative light units (RLU).27
Phytotoxicity assay
In this assay, E-Medium was prepared by mixing a number of constituents in 1L distilled water, the adjustment of pH 6-7 was done by addition of potassium hydroxide pellets (stock solution). The working E-medium was prepared via mixing 900 mL distilled water and 100 mL stock solution. 30 mg of the extract sample was dissolved into 1.5 mL of methanol to make a stock solution of sample. Extract sample was pipetted from stock solution into 3 flasks. The solvent of 3 flasks containing 10, 100 and 1000 μg/mL concentration of extract was evaporated over a night. Working E-medium (20 mL) along with plant Lemna minor (each having rosette of 2 - 3 fronds) were added to every flask (total = 20 fronds). The standard drug (paraquat) was present in other flask supplemented with E-medium. The number of fronds was note down in each flask at 7th day after keeping the flasks in the growth cabinet. The results were evaluated as percentage growth regulation by comparing with negative control.28
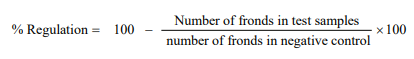
RESULTS
Preliminary phytochemicals screening
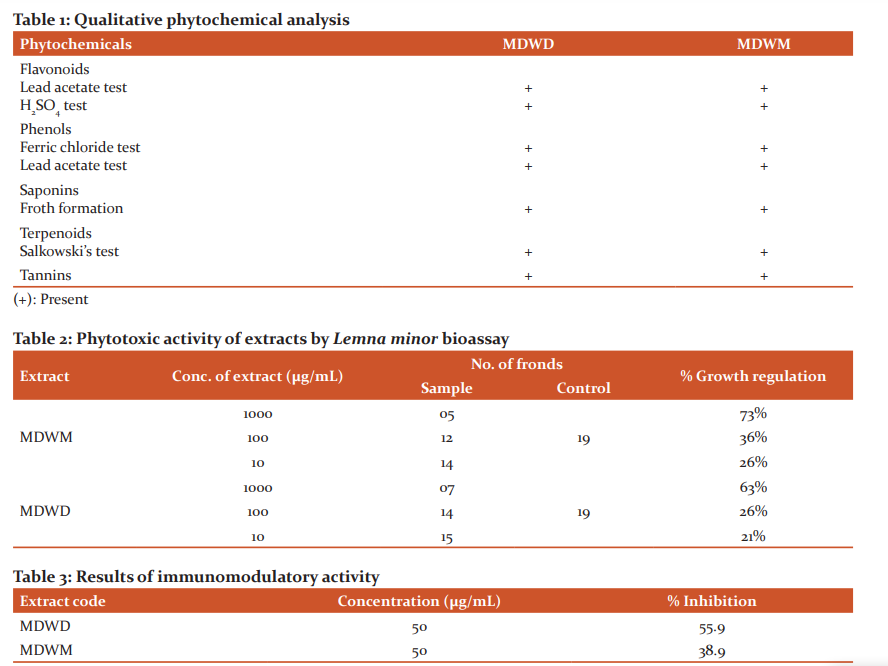
The extracts were preliminarily evaluated for presence of different phytochemical groups with the help of precise chemical-based methods. As indicated by qualitative screening, presence of the terpenoids, tannins, saponins, phenols, and flavonoids was declared in these extracts. The results indicating preliminary phytochemical screening of the extracts are presented in Table 1.
Phytotoxicity bioassay
The phytotoxic capability of dichloromethane and methanol extracts of the whole plant was examined with the help of Lemna minor phytotoxicity bioassay. Growth regulation (%) revealed by crude extracts is presented in Table 2. Results exposed that sample MDWM indicated % growth regulation 26% and 36% at the concentration of 10 and 100µg/mL respectively, but at a concentration of 1000µg/mL, it presented 73% growth regulation. While sample MDWD indicated % growth regulation 21% and 26% at a concentration of a 10 and 100µg/mL respectively, however at a concentration of 1000 µg/mL, it revealed 63% growth regulation. In this way, the extracts were exposed to low growth regulation at concentrations 10 and 100µg/mL, however, appeared to have moderate % growth regulation at high concentration value (1000 µg/mL) by comparing with a standard drug.
Immunomodulatory activity
Luminol-enhanced chemiluminescence assay showed that sample MDWD had moderate activity against ROS (reactive oxygen species) with 55.9% inhibition while sample MDWM showed insignificant immunomodulatory activity with 38.9% inhibition. The results of immunomodulatory activity are given in Table 3. Ibuprofen was used as standard for this assay with % inhibition at 25 µg/mL = 73.2%
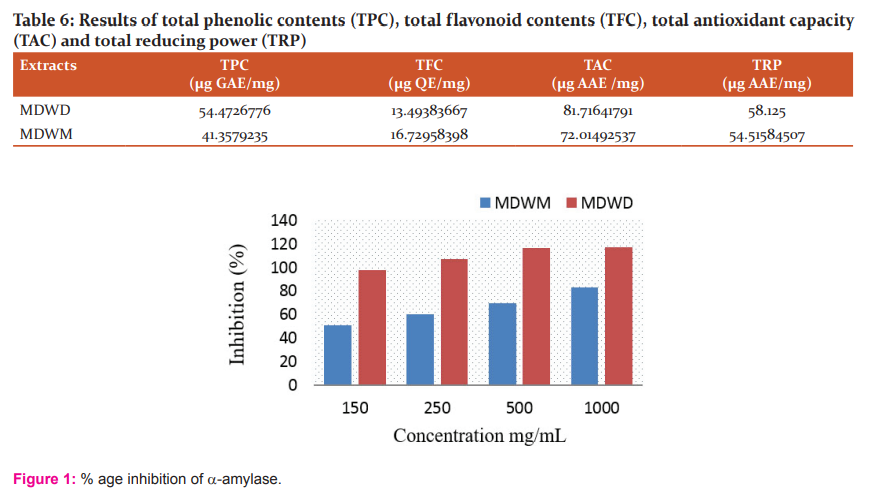
α-amylase inhibitory activity
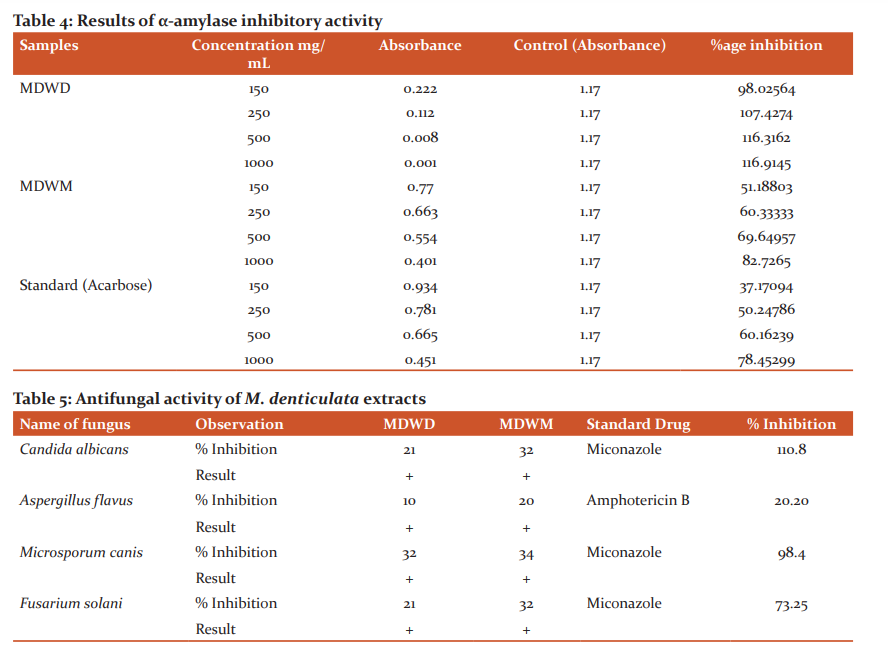
The extracts were also assessed for α-amylase inhibition activity through standard in-vitro α-amylase inhibition assay. Extracts showed highest inhibitory action against ?-amylase. The %age inhibition results of M. denticulata extracts on activity of α-amylase are given in Table 4.
The %age inhibition of ?-amylase increases by increasing concentration of extract as presented in the graph (Figure 1).
Antifungal activity
Antifungal activity of M. denticulata extracts against 4 strains of fungi namelyAspergillus flavus, Fusarium solani, Candida albicans and Microsporum canis is mentioned in Table 5. The information obtained indicates a moderate antifungal action shown by extracts contrary to tested strains. Extracts indicated varying degree of activity against more or less all tested fungi. The maximum inhibition zone was revealed by MDWM extract against Microsporum canis. The effects were compared using standard drugs Amphotericin B and Miconazole. (Table 5)
Total phenolic and flavonoid contents
The recorded values of TFC and TPC in the extracts of M. denticulata are given in Table 6. The total gallic acid equivalent phenol range in the extracts was 41.357 to 54.472 μg GAE/mg DW with maximum content quantified into MDWD extract. Total flavonoid content determination presented that among the extracts, maximum amount of quercetin equivalent flavonoids 16.729 μg QE/mg DW were noted in MDWM extract followed by MDWD extract (13.493μg QE/mg DW).
Total antioxidant capacity (TAC) and total reducing power (TRP)
Phenolic components in several medicinal plants have been demonstrated to be promising contributor towards the antioxidant activity. It is shown that among plant extracts tested, MDWD extract was found to reveal highest antioxidant and reducing potential 81.716µg AAE /mg DW and 58.125 μg AAE/mg DW respectively. The phosphomolybdenum-based antioxidant capacity and reductive power outcomes of Medicago denticulata extracts are given in Table 6.
DISCUSSION
Considering the results of the qualitative screening, these plant extracts are assumed to contain some health-promoting phytochemicals like terpenoids, tannins, saponins, phenols and flavonoids. The presence of these secondary metabolites in various medicinal plants belonging to different families was also reported by.29 The past investigations confirm that Medicago genus is appreciated source of the saponins. Triterpene saponins were recognized in Medicago polymorpha as glycosides of hederagenin, echinocystic acid, bayogenin, caulophyllogenin and soyasapogenol B. The Echinocystic acid, (detected 67.3 ± 0.9% of the total sapogenins), was a main aglycone in Medicago species.30-32
A moderate immunomodulatory activity of dichloromethane extract with 55.9% inhibition was exhibited by the plant. The literature about the reported immunomodulatory activities of medicinal plants supports the current findings. Several plants native to Iran were examined for immunomodulatory properties and at higher concentrations these extracts exhibited peripheral blood lymphocytes (isolated from the healthy individual) inhibitory potential. These inhibitory properties of some plants may be due to the induction of apoptosis.33 The inhibition of ROS generation in whole blood phagocytes by herbal plants has already been reported.34 Methanolic extract of Enicostema axillare acts on cell-mediated and humoral immune functions, reducing release of inflammatory cytokines in peritoneal macrophages.35 An alcoholic extract from the bark of Mangifera indica Linn (having 2.6% mangiferin), was explored for its potential on the humoral and cell-mediated components of mice immune system. It revealed promising immunostimulatory effects.36
M. denticulata has antifungal potential like other Medicago species. The maximum inhibition zone was revealed by MDWM extract against Microsporum canis. Antifungal effect of triterpenoidal glycosides from Medicago sativa has also been previously reported.37
Among plant extracts tested, maximum phenolic contents were quantified into MDWD extract. This extract also revealed highest antioxidant and reducing potential 81.716 µg AAE /mg DW and 58.125 μg AAE/mg DW respectively. A significant correlation among reducing power, antioxidant capacity, and the total phenolic contents were shown in the investigation, signifying that the phenolic compounds may be main contributor towards antioxidant potential of that plant. Numerous classes of polyphenols, including phenolic acids, flavonoids, lignans, and stilbenes present in the medicinal plants had been linked to their antioxidant potentials which are helpful in preventing age-related diseases, mainly produced via oxidative stress.38,39 Thus, currently phenols and flavonoids assurance in these extracts propose this medicinal plant to be a source of valuable natural antioxidants. The previously reported antioxidant activity of Medicago polymorpha extract against free radicals for example DPPH, ABTS and significantly reducing power 40, 41 support the above findings.
Phytotoxicity is an important factor to consider when determining a plant's allelopathic potential. The determination of a plant's phytotoxicity aids in the development of biological herbicides or naturally occuring plant growth regulators. The necessity for phytotoxic compounds cannot be overstated, as the majority of agricultural products are affected by weeds. Human health is negatively impacted by synthetic herbicides. Researchers are battling to find effective, safe and human health-friendly phytotoxic compounds. The occurrence of natural phytotoxic components in the current studied plant make it valuable to isolate important agrochemicals. Phytotoxic effects of crude methanolic extracts of various parts of some medicinal plants on the germination of radish seeds were investigated.42 The phytotoxicity of medicinal plants was also described by.43
The extracts showed a concentration-dependent significant α-amylase inhibitory activity. α-amylase catalyses hydrolysis of 1,4-glycosidic bonds of carbohydrates into monosaccharide single sugar units that can be absorbed more easily from intestine. Inhibition of α- amylase enzyme in human digestive tract is thought to be useful in treating diabetes by reducing of glucose absorption.44 It is indicated from the studies that Azadirachta indica, Mangifera indica and Murraya koenigii are beneficial to manage postprandial hyperglycemia.45 The pancreatic α-amylase inhibitory action of roselle (Hibiscus sabdariffa Linn.) tea extract was described by. 46 α-amylase enzyme was significantly inhibited by Phyllanthus amarus suggesting that the plant contains potential α-amylase inhibitor compounds that may contribute to its antidiabetic activity.47
CONCLUSION
The current study concludes Medicago denticulata as a potential source of phytochemicals. The dichloromethane extract (MDWD) and methanolic extract (MDWM) have potential phenolic and flavonoid contents with important biological activities. Maximum gallic acid equivalent (GAE) total phenolic contents [54.47 and 41.35 μg GAE/mg dry weight] and quercetin equivalent (QE) total flavonoid contents (13.49 and 16.72 μg QE /mg DW) were documented in DCM and methanolic extracts of M. denticulata respectively. Maximum antioxidant capacity represented as ascorbic acid equivalent in DCM and methanol extracts was 81.71 and 72.01 01μg AAE /mg DW respectively, however reducing power noted as ascorbic acid equivalent in DCM and methanol extracts was 58.12 & 54.51 01μg AAE /mg DW. Screened plant extracts are found to have adequate antioxidant potential which may be credited to the existence of flavonoid and phenolic content. The effects of immunomodulatory activity conclude that sample MDWD revealed moderate activity against ROS. The findings also confirm the antifungal, phytotoxic and -amylase inhibitory potential of these extracts. Inhibition of enzyme α-amylase (responsible to hydrolyze carbohydrates) is a significant tactic in lowering postprandial blood glucose level. Obtained results demand further pharmacological description and the bioactivity guided isolation of those components that are responsible for these observed activities.
ACKNOWLEDGEMENTS
The authors would like to thank the Faculty of Pharmacy, Bahauddin Zakariya University Multan, Pakistan for the laboratory facilities to carry out this study.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
SOURCE OF FUNDING
We had no funding sources for this study.
References:
1. Raina H, Soni G, Jauhari N, Sharma N, Bharadvaja N. Phytochemical importance of medicinal plants as potential sources of anticancer agents. Turk J Bot. 2014;38(6):1027-35.
2. Mirzaei-Aghsaghali A. Importance of medical herbs in animal feeding: A review. Ann. Biol. Res. 2012;3(9):918-23.
3. Khan S, Ur-Rehman T, Mirza B, Ul-Haq I, Zia M. Antioxidant, antimicrobial, cytotoxic and protein kinase inhibition activities of fifteen traditional medicinal plants from Pakistan. Pharm. Chem. J. 2017;51(5):391-8.
4. Saad B, Azaizeh H, Said O. Tradition and perspectives of Arab herbal medicine: a review. Evid. Based Complementary Altern. Med. 2005;2(4):475-9.
5. Shoeb M. Anti-cancer agents from medicinal plants. Bangladesh J Pharmacol. 2006;1(2):35-41.
6. Ajose FO. Some Nigerian plants of dermatologic importance. Int. J. Dermatol.. 2007;46:48-55.
7. Harvey AL. Natural products in drug discovery. Drug Discov. Today. 2008;13(19-20):894-901.
8. Meena AK, Bansal P, Kumar S. Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J. Tradit. Med. 2009;4(4):152-70.
9. Bibi Y, Nisa S, Chaudhary FM, Zia M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complement Altern. Med.. 2011;11(1):1-7.
10. Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78(5):431-41.
11. Rehman R, Chaudhary M, Khawar K, Lu G, Mannan A, Zia M. In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia. 2014;69(3):341-9.
12. Alessandra B, Ciccarelli D, Garbari F, Pistelli L. Flavonoids isolated from Medicago littoralis Rhode (Fabaceae): their ecological and them systematic significance. Caryologia. 2010;63(1):106-14.
13. Del Pozo A, Ovalle C, Aronson J, Avendano J. Ecotypic differentiation in Medicago polymorpha L. along an environmental gradient in central Chile. I. Phenology, biomass production and reproductive patterns. Plant Ecol. 2002;159(2):119-30.
14. Abbasi AM, Khan MA, Shah MH, Shah MM, Pervez A, Ahmad M. Ethnobotanical appraisal and cultural values of medicinally important wild edible vegetables of Lesser Himalayas-Pakistan. J. ethnobiol. ethnomed.. 2013;9(1):1-13.
15. Santhi K, Sengottuvel R. Qualitative and quantitative phytochemical analysis of Moringa concanensis Nimmo. Int J Curr Microbiol App Sci. 2016;5(1):633-40.
16. Clarke G, Ting KN, Wiart C, Fry J. High correlation of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants. 2013;2(1):1-10.
17. Tariq H, Zia M, Muhammad SA, Khan SA, Fatima N, Mannan A, et al. Antioxidant, Antimicrobial, Cytotoxic and Protein Kinase Inhibition Potential in Aloe vera L. Biomed Res. Int. 2019;2019(2):1-14.
18. Mannan HA, Ahmed I, Hussain I, Jamil M, Miza B. Antibacterial activity and brine shrimp toxicity of Artemisia dubia extract. Pakistan J. Bot. 2012;44:1487-90.
19. Umamaheswari M, Chatterjee T. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Tradit Complement Altern Med. 2008;5(1):61-73.
20. Afsar T, Razak S, Khan MR, Mawash S, Almajwal A, Shabir M, et al. Evaluation of antioxidant, anti-hemolytic and anticancer activity of various solvent extracts of Acacia hydaspica R. Parker aerial parts. BMC Complement Altern. Med. 2016;16(1):1-16.
21. Jafri L, Saleem S, Ullah N, Mirza B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch. Arab. J. Chem. 2017;10:S3699-S706.
22. Lea C. A note on the chapman and mcfarlane method for the estimation of reducing groups in milk powder. Analyst. 1947;72(857):336-40.
23. Fatima H, Khan K, Zia M, Ur-Rehman T, Mirza B, Haq I-U. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: An in vitro biological and phytochemical investigation. BMC Complement Altern. Med. 2015;15(1):1-18.
24. McCue PP, Shetty K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr. 2004;13(1):101-6.
25. Kazeem M, Adamson J, Ogunwande I. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. Biomed Res. Int.2013;2013:1-6.
26. Maqsood ZT, Khan KM, Ashiq U, Jamal RA, Chohan ZH, Mahroof-Tahir M, et al. Oxovanadium (IV) complexes of hydrazides: potential antifungal agents. J Enzyme Inhib Med Chem. 2006;21(1):37-42.
27. Helfand SL, Werkmeister J, Roder JC. Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. J. Exp. Med. 1982;156(2):492-505.
28. Sharma KR. In-vitro antioxidant, antidiabetic and toxic effect of Ageratum houstonianum from Chitwan district Nepal. Journal of BKC. 2020;9(1):48-54.
29. Doss A. Preliminary phytochemical screening of some Indian medicinal plants. Anc. Sci. Life. 2009;29(2):12.
30. Tava A, Avato P. Chemical and biological activity of triterpene saponins from Medicago species. Nat. Prod. Commun. 2006;1(12):1159-80.
31. Tava A, Pacetti L, Romani M, Mella M, Avato P. Triterpenoid glycosides from the leaves of two cultivars of Medicago polymorpha L. J. Agric. Food Chem. 2011;59(11):6142-9.
32. Tava A, Pecetti L. Chemical investigation of saponins from twelve annual Medicago species and their bioassay with the brine shrimp Artemia salina. Nat. Prod. Commun. 2012;7(7):837-40.
33. Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K, Miri R. Immunomodulatory activities of various medicinal plant extracts: effects on human lymphocytes apoptosis. Immunol. Invest. 2009;38(2):181-92.
34. Ilangkovan M, Jantan I, Mohamad HF, Husain K, Razak A, Faiz A. Inhibitory effects of standardized extracts of Phyllanthus amarus and Phyllanthus urinaria and their marker compounds on phagocytic activity of human neutrophils. Evid.-Based Complementary Altern. Med2013;2013.
35. Saravanan S, Babu NP, Pandikumar P, Raj MK, Paulraj MG, Ignacimuthu S. Immunomodulatory potential of Enicostema axillare (Lam.) A. Raynal, a traditional medicinal plant. J Ethnopharmacol. 2012;140(2):239-46.
36. Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78(2-3):133-7.
37. Abbruscato P, Tosi S, Crispino L, Biazzi E, Menin B, Picco AM, et al. Triterpenoid glycosides from Medicago sativa as antifungal agents against Pyricularia oryzae. J. Agric. Food Chem. 2014;62(46):11030-6.
38. Azwanida N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants. 2015;4(196):1-6.
39. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2(5):270-8.
40. Khan H, Jan SA, Javed M, Shaheen R, Khan Z, Ahmad A, et al. Nutritional composition, antioxidant and antimicrobial activities of selected wild edible plants. J. Food Biochem. 2016;40(1):61-70.
41. Khan MI, Asad S, Zaman G, Rehman H, Rehman S, Iqbal A, et al. Antioxidant And Cytotoxic Activities Of Crude Methanolic Extract Of Medicago Polymorpha. IOSR J. Pharm. 2013;3(8):32-7.
42. Khan AM, Qureshi RA, Ullah F, Gilani SA. Phytotoxic effects of selected medicinal plants collected from Margalla Hills, Islamabad Pakistan. J. Med. Plant Res. 2011;5(18):4671-5.
43. Rauf A, Muhammad N, Khan A, Uddin N, Atif M. Antibacterial and phytotoxic profile of selected Pakistani medicinal plants. World Appl Sci J. 2012;20(4):540-4.
44. Hara Y, Honda M. The inhibition of α-amylase by tea polyphenols. Agric. Biol. Chem. 1990;54(8):1939-45.
45. Dineshkumar B, Mitra A, Manjunatha M. A comparative study of alpha-amylase inhibitory activities of common anti-diabetic plants at Kharagpur 1 block. Int. J. Green Pharm. 2010;4(2):115.
46. Hansawasdi C, Kawabata J, Kasai T. α-Amylase inhibitors from roselle (Hibiscus sabdariffa Linn.) tea. Biosci. Biotechnol. Biochem.. 2000;64(5):1041-3.
47. Ali H, Houghton P, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107(3):449-55.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License