IJCRR - 7(19), October, 2015
Pages: 51-54
Date of Publication: 10-Oct-2015
Print Article
Download XML Download PDF
IDENTIFICATION OF UROVIRULENT MARKERS IN UROPATHOGENIC ESCHERICHIA COLI
Author: D. Vijaya Bharathi, R. Lakshmi Kumari, G. Rajya Lakshmi, Supriya Panda
Category: Healthcare
Abstract:Context: Escherichia coli is the most frequent urinary pathogen isolated from uncomplicated urinary tract infection. These isolates express chromosomally encoded virulence markers. Aims: The present study was designed to determine the urovirulence factors of E.coli isolated from the patients of urinary tract infection and to study their antimicrobial susceptibility pattern. Methods and Material: The study was conducted in the department of microbiology, Rangaraya Medical College, Kakinada, Andhra Pradesh, from October 2010 to September 2011.One hundred and fifty E.coli strains isolated from urine samples and fifty faecal isolates were studied for 1) alpha haemolysin on 5% sheep blood agar, 2) mannose resistant haemagglutination,3) cell surface hydrophobicity, 4) antibiotic susceptibility testing by Kirby-Bauyer's disc diffusion method. Results: Among 150 E.coli isolates from urine tested 82 (54%) and out of 50 E.coli isolates from stool (control), 10 (20%) were positive for virulence markers. In the urinary isolates, the most common virulent marker was Haemolysin 59 (39%), followed by Mannose Resistant Haemagglutination (MRHA) 47(31%) and Cell Surface Hydrophobicity (CSH) 43 (28%). In control group, the occurrence of Haemolysin was 3 (6%), MRHA 6 (12%), CSH 9(18%). The difference between cases and controls for Haemolysin and MRHA were significant. (p< 0.05 and P< 0.005 respectively). Ninety two percent isolates were sensitive to amikacin and high resistance was seen for ampicillin (91%). Conclusions: More number of uropathogenic strains of E. coli expressed urovirulent markers than faecal strains. Majority of the isolates were found to be resistant to routinely used antibiotics.
Keywords: Uropathogenic E. coli, Urovirulent markers, Urinary tract infection
Full Text:
INTRODUCTION
Urinary tract infection is one of the most important causes of morbidity and mortality. Escherichia coli is the most frequent urinary pathogen isolated from 50%-90% of all uncomplicated urinary tract infection [1]. It is now recognized that there are subsets of faecal E.coli which can colonize periurethral area, enter urinary tract and cause symptomatic disease. These are currently defined as Uropathogenic Escherichia coli. It has been traditionally described that certain serotypes of E.coli were consistently associated with uropathogenicity and were designated as uropathogenic E.coli [2]. These isolates express chromosomally encoded virulence markers. The virulence factors include different adhesins, haemolysin production, haemagglutination and cell surface hydrophobicity. Fimbriae mediate the ability of E.coli to adhere to the uroepithelium, thereby resisting elimination by the flow of urine. Adhesion is therefore considered to be an important step in the pathogenesis of urinary tract infection [3]. The studies on the virulence factors of uropathogenic E.coli is limited. So, the present study was designed to determine the urovirulence markers of E.coli isolated from the patients of urinary tract infection and to study their antimicrobial susceptibility pattern.
MATERIALS AND METHODS
The study was conducted in the department of microbiology, Rangaraya Medical College, Kakinada, East Godavari district from October 2010 to September 2011 after taking ethical clearance from Institutional Ethics Committee..One hundred and fifty E.coli strains isolated from urine samples and fifty faecal isolates were studied for 1) alpha haemolysin on 5% sheep blood agar 2) mannose resistant haemagglutination 3) cell surface hydrophobicity 4) antibiotic susceptibility testing by Kirby-Bauyer’s disc diffusion method.
Inclusion criteria
Adult patients with urinary tract infection (UTI) attending various clinical departments of RMC and adult healthy individuals for stool samples are taken.
Samples: 1. Clean-catch midstream urine samples from the patients (n=150) 2. Stool samples of adult healthy individuals (n=50).
Collection and culture of sample: Sample is transported to Microbiology laboratory within half an hour and processed by 1) Wet film preparation for pus cells 2) Culture on Mac Conkey’s agar, Blood agar and Cysteine-Lactose-ElectrolyteDeficient (CLED) agar medium and incubated aerobically at 370 C for 24 hrs 3) Growth on plates and significant bacterial count (i.e. more than 105 colonies / ml of urine) was tested for further identification of E.coli by various biochemical reactions [4] .Such E.coli were screened for virulence markers
Hemolysin: The cytolytic protein secreted by most haemolytic E.coli is known as alpha haemolysin [5]. For detecting haemolysin, 5% sheep blood agar (BA) is used and a zone of alpha haemolysis around each colony is observed after overnight incubation at 370 C aerobically, if haemolysin is produced.
Mannose resistant haemagglutination (HA): HA is detected by clumping of erythrocytes by bacterial fimbriae in the presence of D-Mannose. The test was carried out as per the direct bacterial HA test slide method and mannose sensitive and resistant HA tests [6]. The strains of E.coli were inoculated into 1% nutrient broth and incubated at 37ºc for 48 hours for full fimbriation. Human blood group O+ve red blood cells (RBC) were then washed thrice in normal saline and made up to 3% suspension in fresh saline. They were used immediately or within a week when stored at 3º-5ºc. The slide haemagglutination test was carried out on multiple concavity slide. One drop of RBC suspension was added to a drop of broth culture and slide was rocked to and fro at room temperature for 5 minutes. Presence of clumping was taken as positive for haemagglutination. Control for MSHA is ATCC 25922 and for MRHA in-house control is used. HA was considered to be mannose resistant when it occurred in the presence of 2 % D- mannose and mannose sensitive, when it was inhibited by D-mannose.
Cell surface hydrophobicity: This was done by Salt aggregation test [7]. E.coli grown on Mac Conkey plates was inoculated on to 1ml phosphate buffered saline(PBS) at pH6.8 and turbidity was matched with Mc Farland tubes 6 and 7 to get a colony count 5×109 col/ml. Different molar concentrations of ammonium sulphate 1M,1.4M,2M was prepared. Forty µL PBS at pH 6.8 was taken in first coloumn of VDRL slide. Forty µL of 1M, 1.4M, 2M concentration of ammonium sulphate was taken in each well of other columns of VDRL slide. Forty µL of E.coli suspension is added to each of these wells. The clumps formed in different molar concentration of ammonium sulphate were seen by naked eyes. Positive control used was a strain of E.coli which was haemolytic, MRHA+ve, positive for cell surface hydrophobicity. Negative control used was a strain of E.coli which was non lytic, MRHA-ve, negative for cell surface hydrophobicity. Strains were considered hydrophobic, if they were aggregated in concentration of < 1.4 M. ammonium sulphate.
Antibiotic susceptibility testing: This was performed on all isolates of E.coli by Kirby-Bauer`s disc diffusion method on Muller Hinton agar according to clinical and laboratory standard institute (CLSI) guidelines.
Results:
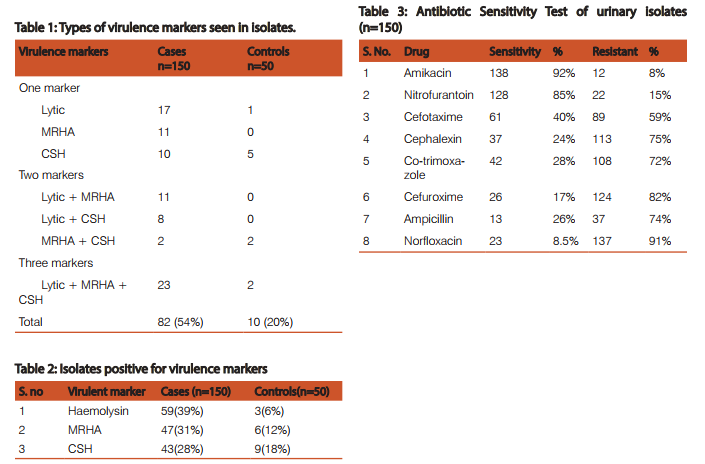
In the present study, out of the 150 patients, 0 – 15 years age group comprised of 42 persons, 16 - 40 years age group comprised of 51 persons and > 40 years group comprised of 57 persons. Females (96) were more than male (54) patients. Among various clinical entities, 84cases presented with lower UTI (56%), 36 cases with asymptomatic bacteriuria (24%), 18 cases presented with renal failure (12%) and 12 with pyelonephritis (8%). Among 150 E.coli isolates from urine tested 82 (54%) were positive for virulence markers and out of 50 E.coli isolates from stool (control), 10 (20%) were positive for virulence markers. Among the urinary isolates tested, the most common virulent marker was Haemolysin 59 (39%), followed by Mannose Resistant Haemagglutination (MRHA) 47(31%) and Cell Surface Hydrophobicity (CSH) 43 (28%). In control group, the occurrence of Haemolysin was 3 (6%), MRHA 6 (12%), CSH 9(18%). There are 38 cases positive for 1 marker, 21 cases positive for 2 markers and 23 cases positive for 3 markers. Ninety two percent isolates were sensitive to amikacin, 85 % to nitrofurantoin, 61 % sensitive to cefotaxime, 28 % to cotrimoxazole. High resistance was seen for ampicillin (91%) followed by norfloxacin (85%) and cefuroxime (82%). Statistical analysis used: Significance of the occurrence of virulence markers in cases and controls was compared by Chi Square test. P value less than 0.05 was considered significant.
Discussion:
Considering the high degree of morbidity in urinary tract infection the uropathogenic E.coli is receiving the more attention and it is important to identify UPEC isolates in the urinary samples [2]. Regarding the positivity of virulence markers, in the present study 54% of urinary isolates were positive for various types of virulence markers indicating their uropathogenicity, whereas 20 % of faecal isolates which were taken as controls were positive for virulence markers (p<0.05) which is statistically significant. Many urinary isolates had shown multiple markers compared to faecal isolates. This clearly shows that presence of virulence markers help in uropathogenicity of E.coli. Similar observation has been reported from India [2,8]. The cytolytic toxin secreted by most haemolytic E coli is hemolysin [5]. E.coli also produces cell associated lysine on blood agar plates and haemolysin causes clear zone of haemolysis [9]. In the present study there was high production of haemolysin in cases when compared to controls and the difference is significant (p<0.05).This is similar to other studies from India [2,10]. Haemagglutination is mediated by P fimbriae [6] and also X, FIC, Dr fimbriae. Thus MRHA positive strains can be considered UPEC most likely having P fimbriae [11]. .In this study there was higher association of MRHA in cases when compared to controls and statistically significant with p value. (p<0.05) and this is similar to studies conducted by Yasmeen Kausar and Prabhat Ranjan et al [8,10]. The surface hydrophobicity is another important virulence factor, which promotes the adherence of the bacteria to various surfaces like the mucosal epithelial cells [12] . In our study 28% E. coli isolates were hydrophobic among cases and 18% among controls with p value more than 0.05 which is statistically insignificant. In the present study, high incidence of UTI was observed in above 40 years age group followed by 16-40 years age group. This is because the elderly patients are likely to be predisposed to conditions like 1. urinary tract obstruction 2. poor bladder emptying 3. diabetes and 4. prostate enlargement in elderly males. These factors favour colonization of bacteria and play an important role in UTI. High incidence of UTI was observed in females (62%) than in males (40%), which is due to 1. short urethra and 2. close proximity of urethra to perianal region. Regarding antibiotic sensitivity test, most of the isolates were sensitive to amikacin (92%) and nitrofurantoin (85%). Whereas most of the isolates were resistant to norfloxacin, ampicillin, cefuroxime, cephalexin, cefotaxime and cotrimoxazole. This shows that aminoglycosides and nitrofurantoin are useful for most of UTI and high level of resistance indicates the lack of rational drug use. In view of emerging resistance, antimicrobial therapy should be started after culture sensitivity report. This will prevent the development of resistance of bacteria towards important antibiotic and we will have choice of antimicrobial agent for complicated urinary tract infection. CONCLUSIONS UTI is one of the most common bacterial infections. UPEC strains display a variety of virulence factors that help to colonize host mucosal surfaces and circumvent host defenses to allow invasion of the normally sterile urinary tract. More number of uropathogenic strains of E. coli expressed urovirulent markers than faecal strains in this study. Pathogenic E.coli strains which express MRHA are more haemolytic and have a high cell surface hydrophobicity which may help them to start an infection. Majority of the isolates were found to be resistant to routinely used antibiotics. These observations emphasize the need of urgent antibiotic policies and infection control measures to be implemented in every hospital.
ACKNOWLEDGEMENT
We acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors /publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Steadman R., and Topley N. 1998. The virulence of Escerichia coli in Urinary tract. Chapter 3 In: Urinary tract infections, 1st ED, Brumfitt W. JeremyMT, Hamilton Miller, Eds, (Chapmanand Hall publication, London). 3741.
2. Raksha R., and Srinivasa, N. 2003. Occurrence and characterization of uropathogenic E.coli in urinary tract infections, Indian. J. Med. Microbiol. 21 (2): 102–107.
3. Struve C., and Krogfelt K.A. 1999. In vivo detection of Escherichia coli type-1 fimbrial expression and phase variation during experimental urinary tract infection. Microbiol. 145:2683-90
4. Cowan and Steel’s Manual for identification of Medical bacteria, 3rd ed. Eds. Barrow Gl, Feltham RKA. Eds. (University of Cambridge) 1993.
5. Cavalieri S.J., Bohach GA and Snyder, I.S. 1984. Escherichia coli alpha haemolysin: characteristics and probable role in pathogenicity. Microbiol. Rev. 48:326-343.
6. Duguid J.P., Clegg S and Wilson MJ.1979. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli . J. Med. Microbiol. 12:213-27.
7. Lindahl M, Faris A, Wadstrom T, Hjerten S. A new test based on salting out to measure relative surface hydrophobicity.
8. Yasmeen Kausar, Sneha K Chunchanur ,Shoba D Nadagir, LH Halesh and MR Chandrasekhar. Virulence factors, Serotypes and antimicrobial susceptibility pattern of Escherichia coli in urinary tract infections.Al Ameen Journal of Medical Sciences(2009) 2(1):47-51
9. Smith,H.W,1963.The hemolysin of Escherichia coli, J Pathol Bact.85:197-211
10. Prabhat Ranjan, K., Neelima Ranjan, Arindam Chakraborthy, D R Arora. 2010. An Approach to Uropathogenic Escherichia coli in Urinary Tract Infections. J. Lab. Physic.2, issue2:70-73.
11. Jonson, J.R. Virulence factors in Escherichia coli urinary tract infections. Clin. Microbiol Rev. 1991. 4:81-128.
12. Mudd S, Mudd EBH. The penetration of bacteria through capillary spaces IV. A kinetic mechanism in interfaces J Exp Med 1924;40:633-45.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License